Murine CMV induces type 1 IFN that impairs differentiation of MDSCs critical for transplantation tolerance
- PMID: 29563123
- PMCID: PMC5873231
- DOI: 10.1182/bloodadvances.2017012187
Murine CMV induces type 1 IFN that impairs differentiation of MDSCs critical for transplantation tolerance
Abstract
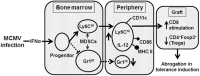
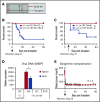
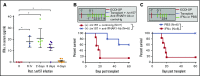
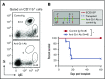
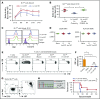
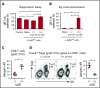
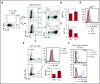
Clinical tolerance without immunosuppression has now been achieved for organ transplantation, and its scope will likely continue to expand. In this context, a previously understudied and now increasingly relevant area is how microbial infections might affect the efficacy of tolerance. A highly prevalent and clinically relevant posttransplant pathogen is cytomegalovirus (CMV). Its impact on transplantation tolerance and graft outcomes is not well defined. Employing a mouse model of CMV (MCMV) infection and allogeneic pancreatic islet transplantation in which donor-specific tolerance was induced by infusing donor splenocytes rendered apoptotic by treatment with ethylenecarbodiimide, we investigated the effect of CMV infection on transplantation tolerance induction. We found that acute MCMV infection abrogated tolerance induction and that this abrogation correlated with an alteration in the differentiation and function of myeloid-derived suppressor cells (MDSCs). These effects on MDSCs were mediated in part through MCMV induced type 1 interferon (IFN) production. During MCMV infection, the highly immunosuppressive Gr1HI-granulocytic MDSCs were markedly reduced in numbers, and the accumulating Ly6CHI-monocytic cells lost their MDSC-like function but instead acquired an immunostimulatory phenotype to cross-present alloantigens and prime alloreactive CD8 T cells. Consequently, the islet allograft exhibited an altered effector to regulatory T-cell ratio that correlated with the ultimate graft demise. Blocking type 1 IFN signaling during MCMV infection rescued MDSC populations and partially restored transplantation tolerance. Our mechanistic studies now provide a solid foundation for seeking effective therapies for promoting transplantation tolerance in settings of CMV infection.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

Similar articles
-
The mTOR Deficiency in Monocytic Myeloid-Derived Suppressor Cells Protects Mouse Cardiac Allografts by Inducing Allograft Tolerance.Front Immunol. 2021 Apr 9;12:661338. doi: 10.3389/fimmu.2021.661338. eCollection 2021. Front Immunol. 2021. PMID: 33897705 Free PMC article.
-
Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes.Immunol Cell Biol. 2017 Jul;95(6):538-548. doi: 10.1038/icb.2017.4. Epub 2017 Jan 21. Immunol Cell Biol. 2017. PMID: 28108746
-
Allogeneic T cells treated with amotosalen prevent lethal cytomegalovirus disease without producing graft-versus-host disease following bone marrow transplantation.J Immunol. 2003 Dec 1;171(11):6023-31. doi: 10.4049/jimmunol.171.11.6023. J Immunol. 2003. PMID: 14634114
-
Myeloid-derived suppressor cells in transplantation tolerance induction.Int Immunopharmacol. 2020 Jun;83:106421. doi: 10.1016/j.intimp.2020.106421. Epub 2020 Mar 24. Int Immunopharmacol. 2020. PMID: 32217462 Review.
-
Myeloid-Derived Suppressor Cells in the Context of Allogeneic Hematopoietic Stem Cell Transplantation.Front Immunol. 2020 May 22;11:989. doi: 10.3389/fimmu.2020.00989. eCollection 2020. Front Immunol. 2020. PMID: 32528476 Free PMC article. Review.
Cited by
-
Impact of infection on transplantation tolerance.Immunol Rev. 2019 Nov;292(1):243-263. doi: 10.1111/imr.12803. Epub 2019 Sep 19. Immunol Rev. 2019. PMID: 31538351 Free PMC article. Review.
-
Myeloid-Derived Suppressor Cells in Lung Transplantation.Front Immunol. 2019 Apr 26;10:900. doi: 10.3389/fimmu.2019.00900. eCollection 2019. Front Immunol. 2019. PMID: 31080450 Free PMC article.
-
New Insights Into the Molecular Mechanisms and Immune Control of Cytomegalovirus Reactivation.Transplantation. 2020 May;104(5):e118-e124. doi: 10.1097/TP.0000000000003138. Transplantation. 2020. PMID: 31996662 Free PMC article. Review.
-
Murine cytomegalovirus dissemination but not reactivation in donor-positive/recipient-negative allogeneic kidney transplantation can be effectively prevented by transplant immune tolerance.Kidney Int. 2020 Jul;98(1):147-158. doi: 10.1016/j.kint.2020.01.034. Epub 2020 Feb 21. Kidney Int. 2020. PMID: 32471635 Free PMC article.
-
In Chronic Hepatitis C Infection, Myeloid-Derived Suppressor Cell Accumulation and T Cell Dysfunctions Revert Partially and Late After Successful Direct-Acting Antiviral Treatment.Front Cell Infect Microbiol. 2019 Jun 14;9:190. doi: 10.3389/fcimb.2019.00190. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31259160 Free PMC article.
References
-
- Fishman JA, Emery V, Freeman R, et al. . Cytomegalovirus in transplantation—challenging the status quo. Clin Transplant. 2007;21(2):149-158. - PubMed
-
- Eid AJ, Razonable RR. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010;70(8):965-981. - PubMed
-
- Stern M, Hirsch H, Cusini A, et al. ; Members of Swiss Transplant Cohort Study. Cytomegalovirus serology and replication remain associated with solid organ graft rejection and graft loss in the era of prophylactic treatment. Transplantation. 2014;98(9):1013-1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

