Angiogenesis Inhibitors for the Treatment of Ovarian Cancer: An Updated Systematic Review and Meta-analysis of Randomized Controlled Trials
- PMID: 29561301
- PMCID: PMC5976222
- DOI: 10.1097/IGC.0000000000001258
Angiogenesis Inhibitors for the Treatment of Ovarian Cancer: An Updated Systematic Review and Meta-analysis of Randomized Controlled Trials
Abstract
Background: Angiogenesis inhibitors showed activity in ovarian cancer, but preliminary data could not accurately reflect the survival benefit. We thus did a systematic review and meta-analysis of randomized controlled trials to reassess the efficacy and safety of angiogenesis inhibitors combined with chemotherapy for ovarian cancer.
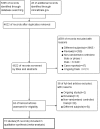
Methods: We searched PubMed, EMBASE, Cochrane, and ClinicalTrials.gov for randomized controlled trials comparing angiogenesis inhibitors containing therapy with conventional chemotherapy alone or no further treatment. Our main outcomes were the progression-free survival (PFS), overall survival (OS), and common adverse events.
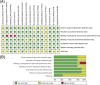
Results: Fifteen trials were included (N = 8721 participants). For newly diagnosed ovarian cancer, combination treatment with angiogenesis inhibitors and chemotherapy yielded a lower risk of disease progression (hazard ratio [HR], 0.83; 95% confidence interval (CI), 0.71-0.97) and no improved OS (HR, 0.95; 95% CI, 0.86-1.05). In the high-risk progression subgroup, the addition of bevacizumab significantly improved PFS (HR, 0.72; 95% CI, 0.65-0.81) and OS (HR, 0.84; 95%CI, 0.74-0.96). In recurrent patients, the combined HR was 0.58 (95% CI, 0.52-0.65) for PFS, and for OS, the combined HR was 0.86 (95% CI, 0.79-0.94). We found no significant improvement for either PFS (HR, 0.80; 95% CI, 0.63-1.01) or OS (HR, 1.06; 95% CI, 0.88-1.28) in the pure maintenance therapy.In the overall population, angiogenesis inhibitors increased the incidence of gastrointestinal perforation (risk ratio [RR], 2.57; 95% CI, 1.66-3.97), hypertension (RR, 7.60; 95% CI, 2.79-20.70), arterial thromboembolism (RR, 2.27; 95% CI, 1.34-3.84), proteinuria (RR, 4.31; 95% CI, 2.15-8.64), and complication of wound healing (RR, 1.72, 95% CI, 1.12-2.63).
Conclusions: Combination treatment with angiogenesis inhibitors and chemotherapy significantly improved PFS and OS in both patients with high-risk of progression and recurrent ovarian cancer, with an increased incidence of common adverse events. Conversely, we detected no statistically significant survival benefit in the pure maintenance setting. The main limitation of the review is clinical heterogeneity across the studies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Bevacizumab combined with chemotherapy for ovarian cancer: an updated systematic review and meta-analysis of randomized controlled trials.Oncotarget. 2017 Feb 7;8(6):10703-10713. doi: 10.18632/oncotarget.12926. Oncotarget. 2017. PMID: 27793044 Free PMC article. Review.
-
Angiogenesis inhibitors for the treatment of epithelial ovarian cancer.Cochrane Database Syst Rev. 2023 Apr 18;4(4):CD007930. doi: 10.1002/14651858.CD007930.pub3. Cochrane Database Syst Rev. 2023. PMID: 37185961 Free PMC article. Review.
-
The efficacy and toxicity of angiogenesis inhibitors for ovarian cancer: a meta-analysis of randomized controlled trials.Arch Gynecol Obstet. 2021 Feb;303(2):285-311. doi: 10.1007/s00404-020-05865-z. Epub 2020 Nov 21. Arch Gynecol Obstet. 2021. PMID: 33222040 Review.
-
Angiogenesis inhibitors for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2011 Sep 7;(9):CD007930. doi: 10.1002/14651858.CD007930.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2023 Apr 18;4:CD007930. doi: 10.1002/14651858.CD007930.pub3 PMID: 21901715 Free PMC article. Updated. Review.
-
Addition of bevacizumab to chemotherapy in patients with ovarian cancer: a systematic review and meta-analysis of randomized trials.Clin Transl Oncol. 2015 Sep;17(9):673-83. doi: 10.1007/s12094-015-1293-z. Epub 2015 May 20. Clin Transl Oncol. 2015. PMID: 25990506 Review.
Cited by
-
Metabolomic Analysis of Histological Composition Variability of High-Grade Serous Ovarian Cancer Using 1H HR MAS NMR Spectroscopy.Int J Mol Sci. 2024 Oct 10;25(20):10903. doi: 10.3390/ijms252010903. Int J Mol Sci. 2024. PMID: 39456684 Free PMC article.
-
Bevacizumab Combined with Chemotherapy in Platinum-Resistant Ovarian Cancer: Beyond the AURELIA Trial.Transl Cancer Res. 2020 Apr;9(4):2164-2167. doi: 10.21037/tcr.2020.02.42. Transl Cancer Res. 2020. PMID: 34113549 Free PMC article. No abstract available.
-
Stiffness increases with myofibroblast content and collagen density in mesenchymal high grade serous ovarian cancer.Sci Rep. 2021 Feb 18;11(1):4219. doi: 10.1038/s41598-021-83685-0. Sci Rep. 2021. PMID: 33603134 Free PMC article.
-
Theasaponin E1 Inhibits Platinum-Resistant Ovarian Cancer Cells through Activating Apoptosis and Suppressing Angiogenesis.Molecules. 2021 Mar 17;26(6):1681. doi: 10.3390/molecules26061681. Molecules. 2021. PMID: 33802884 Free PMC article.
-
Efficacy and safety of VEGFR inhibitors for recurrent ovarian cancer: a systematic review.Future Oncol. 2024;20(26):1943-1960. doi: 10.1080/14796694.2024.2373680. Epub 2024 Aug 12. Future Oncol. 2024. PMID: 39129672
References
-
- Holmes D. Ovarian cancer: beyond resistance. . 2015;527:S217 doi:10.1038/527S217a. - PubMed
-
- Swisher EM, Lin KK, Oza AM, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. . 2017;18:75–87. - PubMed
-
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. . 2011;365:2473–2483. - PubMed
-
- Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. . 2014;384:1376–1388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
