Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing
- PMID: 29545604
- PMCID: PMC5992128
- DOI: 10.1038/s41388-018-0209-0
Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing
Abstract
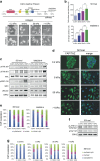
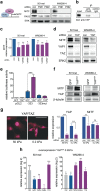
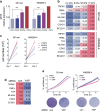
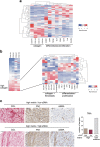
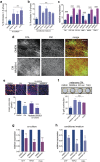
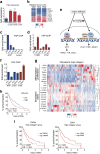
Despite the general focus on an invasive and de-differentiated phenotype as main driver of cancer metastasis, in melanoma patients many metastatic lesions display a high degree of pigmentation, indicative for a differentiated phenotype. Indeed, studies in mice and fish show that melanoma cells switch to a differentiated phenotype at secondary sites, possibly because in melanoma differentiation is closely linked to proliferation through the lineage-specific transcriptional master regulator MITF. Importantly, while a lot of effort has gone into identifying factors that induce the de-differentiated/invasive phenotype, it is not well understood how the switch to the differentiated/proliferative phenotype is controlled. We identify collagen as a contributor to this switch. We demonstrate that collagen stiffness induces melanoma differentiation through a YAP/PAX3/MITF axis and show that in melanoma patients increased collagen abundance correlates with nuclear YAP localization. However, the interrogation of large patient datasets revealed that in the context of the tumour microenvironment, YAP function is more complex. In the absence of fibroblasts, YAP/PAX3-mediated transcription prevails, but in the presence of fibroblasts tumour growth factor-β suppresses YAP/PAX3-mediated MITF expression and induces YAP/TEAD/SMAD-driven transcription and a de-differentiated phenotype. Intriguingly, while high collagen expression is correlated with poorer patient survival, the worst prognosis is seen in patients with high collagen expression, who also express MITF target genes such as the differentiation markers TRPM1, TYR and TYRP1, as well as CDK4. In summary, we reveal a distinct lineage-specific route of YAP signalling that contributes to the regulation of melanoma pigmentation and uncovers a set of potential biomarkers predictive for poor survival.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Nuclear Localization of Yes-Associated Protein Is Associated With Tumor Progression in Cutaneous Melanoma.Lab Invest. 2024 May;104(5):102048. doi: 10.1016/j.labinv.2024.102048. Epub 2024 Mar 14. Lab Invest. 2024. PMID: 38490470
-
Heterogeneous SWI/SNF chromatin remodeling complexes promote expression of microphthalmia-associated transcription factor target genes in melanoma.Oncogene. 2010 Jan 7;29(1):81-92. doi: 10.1038/onc.2009.304. Epub 2009 Sep 28. Oncogene. 2010. PMID: 19784067 Free PMC article.
-
PAX3 knockdown in metastatic melanoma cell lines does not reduce MITF expression.Melanoma Res. 2011 Feb;21(1):24-34. doi: 10.1097/CMR.0b013e328341c7e0. Melanoma Res. 2011. PMID: 21164369
-
MITF, the Janus transcription factor of melanoma.Future Oncol. 2013 Feb;9(2):235-44. doi: 10.2217/fon.12.177. Future Oncol. 2013. PMID: 23414473 Review.
-
Microphthalmia-associated transcription factor expression levels in melanoma cells contribute to cell invasion and proliferation.Exp Dermatol. 2015 Jul;24(7):481-4. doi: 10.1111/exd.12724. Exp Dermatol. 2015. PMID: 25866058 Review.
Cited by
-
Osteoblasts contribute to a protective niche that supports melanoma cell proliferation and survival.Pigment Cell Melanoma Res. 2020 Jan;33(1):74-85. doi: 10.1111/pcmr.12812. Epub 2019 Aug 8. Pigment Cell Melanoma Res. 2020. PMID: 31323160 Free PMC article.
-
The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment.Cancers (Basel). 2024 Feb 23;16(5):913. doi: 10.3390/cancers16050913. Cancers (Basel). 2024. PMID: 38473275 Free PMC article. Review.
-
Fibroblast MMP14-Dependent Collagen Processing Is Necessary for Melanoma Growth.Cancers (Basel). 2021 Apr 20;13(8):1984. doi: 10.3390/cancers13081984. Cancers (Basel). 2021. PMID: 33924099 Free PMC article.
-
Low-Temperature Argon Plasma Regulates Skin Moisturizing and Melanogenesis-Regulating Markers through Yes-Associated Protein.Int J Mol Sci. 2021 Feb 14;22(4):1895. doi: 10.3390/ijms22041895. Int J Mol Sci. 2021. PMID: 33672928 Free PMC article.
-
Epigenetic Mechanisms Underlying Melanoma Resistance to Immune and Targeted Therapies.Cancers (Basel). 2022 Nov 28;14(23):5858. doi: 10.3390/cancers14235858. Cancers (Basel). 2022. PMID: 36497341 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

