Rapid production of pure recombinant actin isoforms in Pichia pastoris
- PMID: 29535210
- PMCID: PMC5976186
- DOI: 10.1242/jcs.213827
Rapid production of pure recombinant actin isoforms in Pichia pastoris
Abstract
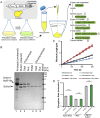
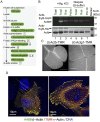
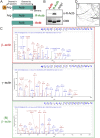
Actins are major eukaryotic cytoskeletal proteins, and they are involved in many important cell functions, including cell division, cell polarity, wound healing and muscle contraction. Despite obvious drawbacks, muscle actin, which is easily purified, is used extensively for biochemical studies of the non-muscle actin cytoskeleton. Here, we report a rapid and cost-effective method to purify heterologous actins expressed in the yeast Pichia pastoris Actin is expressed as a fusion with the actin-binding protein thymosin β4 and purified by means of an affinity tag introduced in the fusion. Following cleavage of thymosin β4 and the affinity tag, highly purified functional full-length actin is liberated. We purify actins from Saccharomycescerevisiae and Schizosaccharomycespombe, and the β- and γ-isoforms of human actin. We also report a modification of the method that facilitates expression and purification of arginylated actin, a form of actin thought to regulate dendritic actin networks in mammalian cells. The methods we describe can be performed in all laboratories equipped for molecular biology, and should greatly facilitate biochemical and cell biological studies of the actin cytoskeleton.
Keywords: Actin; Actin purification; Biochemistry; Cytoskeleton.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Pick-ya actin - a method to purify actin isoforms with bespoke key post-translational modifications.J Cell Sci. 2020 Jan 30;133(2):jcs241406. doi: 10.1242/jcs.241406. J Cell Sci. 2020. PMID: 31964701 Free PMC article.
-
C-terminal variations in beta-thymosin family members specify functional differences in actin-binding properties.J Cell Biochem. 2000 Mar;77(2):277-87. doi: 10.1002/(sici)1097-4644(20000501)77:2<277::aid-jcb10>3.0.co;2-q. J Cell Biochem. 2000. PMID: 10723093
-
Expression of recombinant GFP-actin fusion protein in the methylotrophic yeast Pichia pastoris.FEMS Yeast Res. 2003 Mar;3(1):105-11. doi: 10.1016/s1567-1356(02)00160-5. FEMS Yeast Res. 2003. PMID: 12702253
-
Reconsidering an active role for G-actin in cytoskeletal regulation.J Cell Sci. 2018 Jan 10;131(1):jcs203760. doi: 10.1242/jcs.203760. J Cell Sci. 2018. PMID: 29321224 Free PMC article. Review.
-
Are non-muscle actin isoforms functionally equivalent?Histol Histopathol. 2017 Nov;32(11):1125-1139. doi: 10.14670/HH-11-896. Epub 2017 Apr 25. Histol Histopathol. 2017. PMID: 28439872 Review.
Cited by
-
Specialization of actin isoforms derived from the loss of key interactions with regulatory factors.EMBO J. 2022 Mar 1;41(5):e107982. doi: 10.15252/embj.2021107982. Epub 2022 Feb 18. EMBO J. 2022. PMID: 35178724 Free PMC article.
-
Identification of the Actin-Binding Region and Binding to Host Plant Apple Actin of Immunodominant Transmembrane Protein of 'Candidatus Phytoplasma mali'.Int J Mol Sci. 2023 Jan 4;24(2):968. doi: 10.3390/ijms24020968. Int J Mol Sci. 2023. PMID: 36674483 Free PMC article.
-
IntAct: A nondisruptive internal tagging strategy to study the organization and function of actin isoforms.PLoS Biol. 2024 Mar 11;22(3):e3002551. doi: 10.1371/journal.pbio.3002551. eCollection 2024 Mar. PLoS Biol. 2024. PMID: 38466773 Free PMC article.
-
Purification of human β- and γ-actin from budding yeast.J Cell Sci. 2023 May 1;136(9):jcs260540. doi: 10.1242/jcs.260540. Epub 2023 May 9. J Cell Sci. 2023. PMID: 37070275 Free PMC article.
-
Tools of the trade: studying actin in zebrafish.Histochem Cell Biol. 2020 Nov;154(5):481-493. doi: 10.1007/s00418-020-01932-3. Epub 2020 Oct 23. Histochem Cell Biol. 2020. PMID: 33095903 Free PMC article. Review.
References
-
- Cook R. K., Blake W. T. and Rubenstein P. A. (1992). Removal of the amino-terminal acidic residues of yeast actin. Studies in vitro and in vivo. 267, 9430-9436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

