TRPA1 and CGRP antagonists counteract vesicant-induced skin injury and inflammation
- PMID: 29535050
- PMCID: PMC5975083
- DOI: 10.1016/j.toxlet.2018.03.007
TRPA1 and CGRP antagonists counteract vesicant-induced skin injury and inflammation
Abstract
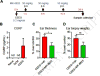
The skin is highly sensitive to the chemical warfare agent in mustard gas, sulfur mustard (SM) that initiates a delayed injury response characterized by erythema, inflammation and severe vesication (blistering). Although SM poses a continuing threat, used as recently as in the Syrian conflict, no mechanism-based antidotes against SM are available. Recent studies demonstrated that Transient Receptor Potential Ankyrin 1 (TRPA1), a chemosensory cation channel in sensory nerves innervating the skin, is activated by SM and 2-chloroethyl ethyl sulfide (CEES), an SM analog, in vitro, suggesting it may promote vesicant injury. Here, we investigated the effects of TRPA1 inhibitors, and an inhibitor of Calcitonin Gene Related Peptide (CGRP), a neurogenic inflammatory peptide released upon TRPA1 activation, in a CEES-induced mouse ear vesicant model (CEES-MEVM). TRPA1 inhibitors (HC-030031 and A-967079) and a CGRP inhibitor (MK-8825) reduced skin edema, pro-inflammatory cytokines (IL-1β, CXCL1/KC), MMP-9, a protease implicated in skin damage, and improved histopathological outcomes. These findings suggest that TRPA1 and neurogenic inflammation contribute to the deleterious effects of vesicants in vivo, activated either directly by alkylation, or indirectly, by reactive intermediates or pro-inflammatory mediators. TRPA1 and CGRP inhibitors represent new leads that could be considered for validation and further development in other vesicant injury models.
Keywords: CEES; CGRP; Medical countermeasures; Mouse ear vesicant model (MEVM); Skin vesicant injury; Sulfur mustard; TRPA1.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Sven-Eric Jordt is serving on the Scientific Advisory Board of Hydra Biosciences Inc., a biopharmaceutical company developing TRP ion channel inhibitors for the treatment of pain and inflammation.
Figures

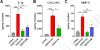

Similar articles
-
Sulfur mustard analog, 2-chloroethyl ethyl sulfide-induced skin injury involves DNA damage and induction of inflammatory mediators, in part via oxidative stress, in SKH-1 hairless mouse skin.Toxicol Lett. 2011 Sep 10;205(3):293-301. doi: 10.1016/j.toxlet.2011.06.019. Epub 2011 Jun 21. Toxicol Lett. 2011. PMID: 21722719 Free PMC article.
-
Expression of cytokines and chemokines in mouse skin treated with sulfur mustard.Toxicol Appl Pharmacol. 2018 Sep 15;355:52-59. doi: 10.1016/j.taap.2018.06.008. Epub 2018 Jun 20. Toxicol Appl Pharmacol. 2018. PMID: 29935281 Free PMC article.
-
Activation of the chemosensing transient receptor potential channel A1 (TRPA1) by alkylating agents.Arch Toxicol. 2015 Sep;89(9):1631-43. doi: 10.1007/s00204-014-1414-4. Epub 2014 Nov 14. Arch Toxicol. 2015. PMID: 25395009
-
Sulfur mustard skin lesions: A systematic review on pathomechanisms, treatment options and future research directions.Toxicol Lett. 2018 Sep 1;293:82-90. doi: 10.1016/j.toxlet.2017.11.039. Epub 2017 Dec 2. Toxicol Lett. 2018. PMID: 29203275 Review.
-
Acute and chronic effects of sulfur mustard on the skin: a comprehensive review.Cutan Ocul Toxicol. 2010 Dec;29(4):269-77. doi: 10.3109/15569527.2010.511367. Epub 2010 Sep 24. Cutan Ocul Toxicol. 2010. PMID: 20868209 Review.
Cited by
-
Transient receptor potential ankyrin 1 (TRPA1) positively regulates imiquimod-induced, psoriasiform dermal inflammation in mice.J Cell Mol Med. 2019 Jul;23(7):4819-4828. doi: 10.1111/jcmm.14392. Epub 2019 May 21. J Cell Mol Med. 2019. PMID: 31111624 Free PMC article.
-
Pharmacologic Inhibition of Transient Receptor Potential Ion Channel Ankyrin 1 Counteracts 2-Chlorobenzalmalononitrile Tear Gas Agent-Induced Cutaneous Injuries.J Pharmacol Exp Ther. 2024 Jan 17;388(2):613-623. doi: 10.1124/jpet.123.001666. J Pharmacol Exp Ther. 2024. PMID: 38050077 Free PMC article.
-
Crotonaldehyde-induced vascular relaxation and toxicity: Role of endothelium and transient receptor potential ankyrin-1 (TRPA1).Toxicol Appl Pharmacol. 2020 Jul 1;398:115012. doi: 10.1016/j.taap.2020.115012. Epub 2020 Apr 19. Toxicol Appl Pharmacol. 2020. PMID: 32320793 Free PMC article.
-
Wound Repair and Ca2+ Signalling Interplay: The Role of Ca2+ Channels in Skin.Cells. 2024 Mar 11;13(6):491. doi: 10.3390/cells13060491. Cells. 2024. PMID: 38534335 Free PMC article. Review.
-
TRPs in Tox: Involvement of Transient Receptor Potential-Channels in Chemical-Induced Organ Toxicity-A Structured Review.Cells. 2018 Aug 7;7(8):98. doi: 10.3390/cells7080098. Cells. 2018. PMID: 30087301 Free PMC article. Review.
References
-
- Alizadeh AH, Ranjbar M, Yadollahzadeh M. Patient concerns regarding chronic hepatitis B and C infection. East Mediterr Health J. 2008;14:1142–1147. - PubMed
-
- Babin MC, Ricketts K, Skvorak JP, Gazaway M, Mitcheltree LW, Casillas RP. Systemic administration of candidate antivesicants to protect against topically applied sulfur mustard in the mouse ear vesicant model (MEVM) J Appl Toxicol. 2000;20(Suppl 1):S141–144. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

