Phagocytosis depends on TRPV2-mediated calcium influx and requires TRPV2 in lipids rafts: alteration in macrophages from patients with cystic fibrosis
- PMID: 29523858
- PMCID: PMC5844937
- DOI: 10.1038/s41598-018-22558-5
Phagocytosis depends on TRPV2-mediated calcium influx and requires TRPV2 in lipids rafts: alteration in macrophages from patients with cystic fibrosis
Abstract
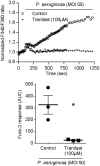
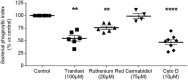
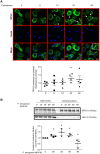
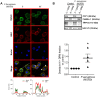
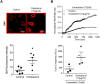
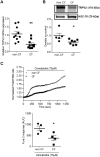
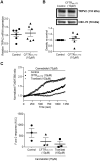
Whereas many phagocytosis steps involve ionic fluxes, the underlying ion channels remain poorly defined. As reported in mice, the calcium conducting TRPV2 channel impacts the phagocytic process. Macrophage phagocytosis is critical for defense against pathogens. In cystic fibrosis (CF), macrophages have lost their capacity to act as suppressor cells and thus play a significant role in the initiating stages leading to chronic inflammation/infection. In a previous study, we demonstrated that impaired function of CF macrophages is due to a deficient phagocytosis. The aim of the present study was to investigate TRPV2 role in the phagocytosis capacity of healthy primary human macrophage by studying its activity, its membrane localization and its recruitment in lipid rafts. In primary human macrophages, we showed that P. aeruginosa recruits TRPV2 channels at the cell surface and induced a calcium influx required for bacterial phagocytosis. We presently demonstrate that to be functional and play a role in phagocytosis, TRPV2 might require a preferential localization in lipid rafts. Furthermore, CF macrophage displays a perturbed calcium homeostasis due to a defect in TRPV2. In this context, deregulated TRPV2-signaling in CF macrophages could explain their defective phagocytosis capacity that contribute to the maintenance of chronic infection.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
TRPV2 has a pivotal role in macrophage particle binding and phagocytosis.Nat Immunol. 2010 Mar;11(3):232-9. doi: 10.1038/ni.1842. Epub 2010 Jan 31. Nat Immunol. 2010. PMID: 20118928 Free PMC article.
-
Reduced transient receptor potential vanilloid 2 expression in alveolar macrophages causes COPD in mice through impaired phagocytic activity.BMC Pulm Med. 2019 Mar 26;19(1):70. doi: 10.1186/s12890-019-0821-y. BMC Pulm Med. 2019. PMID: 30914062 Free PMC article.
-
Nitric oxide upregulates microglia phagocytosis and increases transient receptor potential vanilloid type 2 channel expression on the plasma membrane.Glia. 2019 Dec;67(12):2294-2311. doi: 10.1002/glia.23685. Epub 2019 Aug 27. Glia. 2019. PMID: 31453646
-
Potential roles in cardiac physiology and pathology of the cation channel TRPV2 expressed in cardiac cells and cardiac macrophages: a mini-review.Am J Physiol Heart Circ Physiol. 2020 Jan 1;318(1):H181-H188. doi: 10.1152/ajpheart.00491.2019. Epub 2019 Dec 6. Am J Physiol Heart Circ Physiol. 2020. PMID: 31809212 Review.
-
The impact of impaired macrophage functions in cystic fibrosis disease progression.J Cyst Fibros. 2017 Jul;16(4):443-453. doi: 10.1016/j.jcf.2016.10.011. Epub 2016 Nov 14. J Cyst Fibros. 2017. PMID: 27856165 Review.
Cited by
-
Cystic fibrosis macrophage function and clinical outcomes after elexacaftor/tezacaftor/ivacaftor.Eur Respir J. 2023 Apr 1;61(4):2102861. doi: 10.1183/13993003.02861-2021. Print 2023 Apr. Eur Respir J. 2023. PMID: 36265882 Free PMC article.
-
Fine Tuning of Calcium Constitutive Entry by Optogenetically-Controlled Membrane Polarization: Impact on Cell Migration.Cells. 2020 Jul 13;9(7):1684. doi: 10.3390/cells9071684. Cells. 2020. PMID: 32668787 Free PMC article.
-
Immunomodulation in Cystic Fibrosis: Why and How?Int J Mol Sci. 2020 May 8;21(9):3331. doi: 10.3390/ijms21093331. Int J Mol Sci. 2020. PMID: 32397175 Free PMC article. Review.
-
Ion channel TRPV2 is critical in enhancing B cell activation and function.J Exp Med. 2024 Mar 4;221(3):e20221042. doi: 10.1084/jem.20221042. Epub 2024 Feb 14. J Exp Med. 2024. PMID: 38353705 Free PMC article.
-
The TRPV2 cation channels: from urothelial cancer invasiveness to glioblastoma multiforme interactome signature.Lab Invest. 2020 Feb;100(2):186-198. doi: 10.1038/s41374-019-0333-7. Epub 2019 Oct 25. Lab Invest. 2020. PMID: 31653969 Review.
References
-
- Carrithers MD, et al. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J. Immunol. Baltim. Md 1950. 2007;178:7822–7832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

