Oxidative Stress and NLRP3-Inflammasome Activity as Significant Drivers of Diabetic Cardiovascular Complications: Therapeutic Implications
- PMID: 29515457
- PMCID: PMC5826188
- DOI: 10.3389/fphys.2018.00114
Oxidative Stress and NLRP3-Inflammasome Activity as Significant Drivers of Diabetic Cardiovascular Complications: Therapeutic Implications
Abstract
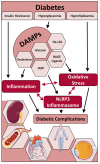
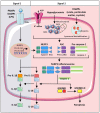
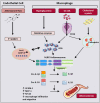
It is now increasingly appreciated that inflammation is not limited to the control of pathogens by the host, but rather that sterile inflammation which occurs in the absence of viral or bacterial pathogens, accompanies numerous disease states, none more so than the complications that arise as a result of hyperglycaemia. Individuals with type 1 or type 2 diabetes mellitus (T1D, T2D) are at increased risk of developing cardiac and vascular complications. Glucose and blood pressure lowering therapies have not stopped the advance of these morbidities that often lead to fatal heart attacks and/or stroke. A unifying mechanism of hyperglycemia-induced cellular damage was initially proposed to link elevated blood glucose levels with oxidative stress and the dysregulation of metabolic pathways. Pre-clinical evidence has, in most cases, supported this notion. However, therapeutic strategies to lessen oxidative stress in clinical trials has not proved efficacious, most likely due to indiscriminate targeting by antioxidants such as vitamins. Recent evidence now suggests that oxidative stress is a major driver of inflammation and vice versa, with the latest findings suggesting not only a key role for inflammatory pathways underpinning metabolic and haemodynamic dysfunction in diabetes, but furthermore that these perturbations are driven by activation of the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome. This review will address these latest findings with an aim of highlighting the interconnectivity between oxidative stress, NLRP3 activation and inflammation as it pertains to cardiac and vascular injury sustained by diabetes. Current therapeutic strategies to lessen both oxidative stress and inflammation will be emphasized. This will be placed in the context of improving the burden of these diabetic complications.
Keywords: NLRP3 inflammasome; diabetic atherosclerosis; diabetic cardiomyopathy; diabetic complications; diabetic nephropathy; inflammation; inflammatory cytokines; oxidative stress.
Figures
Similar articles
-
The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation.J Pathol. 2013 Nov;231(3):342-53. doi: 10.1002/path.4237. Epub 2013 Sep 3. J Pathol. 2013. PMID: 23843215
-
Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-κB and NLRP3 inflammasome pathways.Biomed Pharmacother. 2018 Oct;106:976-982. doi: 10.1016/j.biopha.2018.07.045. Epub 2018 Jul 14. Biomed Pharmacother. 2018. PMID: 30119269
-
Mitochondria, the NLRP3 Inflammasome, and Sirtuins in Type 2 Diabetes: New Therapeutic Targets.Antioxid Redox Signal. 2018 Sep 10;29(8):749-791. doi: 10.1089/ars.2017.7313. Epub 2018 Jan 30. Antioxid Redox Signal. 2018. PMID: 29256638 Review.
-
Pterostilbene Decreases Cardiac Oxidative Stress and Inflammation via Activation of AMPK/Nrf2/HO-1 Pathway in Fructose-Fed Diabetic Rats.Cardiovasc Drugs Ther. 2018 Apr;32(2):147-163. doi: 10.1007/s10557-018-6780-3. Cardiovasc Drugs Ther. 2018. PMID: 29556862
-
NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases.Redox Biol. 2015;4:296-307. doi: 10.1016/j.redox.2015.01.008. Epub 2015 Jan 14. Redox Biol. 2015. PMID: 25625584 Free PMC article. Review.
Cited by
-
The Role of NLRP3 Inflammasome Signaling on Arrhythmias in Diabetes.J Inflamm Res. 2022 Dec 29;15:6883-6889. doi: 10.2147/JIR.S390310. eCollection 2022. J Inflamm Res. 2022. PMID: 36600995 Free PMC article. Review.
-
Targeting NLRP3 Inflammasome in the Treatment of CNS Diseases.Front Mol Neurosci. 2018 Sep 4;11:320. doi: 10.3389/fnmol.2018.00320. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30233319 Free PMC article. Review.
-
Pharmacological Basis for Use of a Novel Compound in Hyperuricemia: Anti-Hyperuricemic and Anti-Inflammatory Effects.Front Pharmacol. 2021 Nov 8;12:772504. doi: 10.3389/fphar.2021.772504. eCollection 2021. Front Pharmacol. 2021. PMID: 34819865 Free PMC article.
-
Insight into Cardioprotective Effects and Mechanisms of Dexmedetomidine.Cardiovasc Drugs Ther. 2024 Dec;38(6):1139-1159. doi: 10.1007/s10557-024-07579-9. Epub 2024 Jun 13. Cardiovasc Drugs Ther. 2024. PMID: 38869744 Review.
-
Hydrogen Sulfide Plays an Important Role in Diabetic Cardiomyopathy.Front Cell Dev Biol. 2021 Feb 18;9:627336. doi: 10.3389/fcell.2021.627336. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33681206 Free PMC article. Review.
References
-
- Abderrazak A., Couchie D., Mahmood D. F., Elhage R., Vindis C., Laffargue M., et al. . (2015). Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 131, 1061–1070. 10.1161/CIRCULATIONAHA.114.013730 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

