Tumor oxygenation and cancer therapy-then and now
- PMID: 29513032
- PMCID: PMC6435050
- DOI: 10.1259/bjr.20170955
Tumor oxygenation and cancer therapy-then and now
Abstract
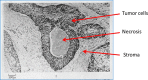
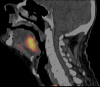
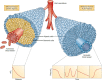
In 2012, cancer affected 14.1 million people worldwide and was responsible for 8.2 million deaths. The disease predominantly affects aged populations and is one of the leading causes of death in most western countries. In tumors, the aggressive growth of the neoplastic cell population and associated overexpression of pro-angiogenic factors lead to the development of disorganized blood vessel networks that are structurally and functionally different from normal vasculature. A disorganized labyrinth of vessels that are immature, tortuous and hyperpermeable typifies tumor vasculature. Functionally, the ability of the tumor vasculature to deliver nutrients and remove waste products is severely diminished. A critical consequence of the inadequate vascular networks in solid tumors is the development of regions of hypoxia [low oxygen tensions typically defined as oxygen tensions (pO2 values) < 10 mm Hg]. Tumor cells existing in such hypoxic environments have long been known to be resistant to anticancer therapy, display an aggressive phenotype, and promote tumor progression and dissemination. This review discusses the physiological basis of hypoxia, methods of detection, and strategies to overcome the resulting therapy resistance.
Figures


Similar articles
-
[Anemia and radiotherapy].Bull Cancer. 2003 Apr;90 Spec No:S152-7. Bull Cancer. 2003. PMID: 12856427 Review. French.
-
Treatment resistance of solid tumors: role of hypoxia and anemia.Med Oncol. 2001;18(4):243-59. doi: 10.1385/MO:18:4:243. Med Oncol. 2001. PMID: 11918451 Review.
-
Modulation of cell death in the tumor microenvironment.Semin Radiat Oncol. 2003 Jan;13(1):31-41. doi: 10.1053/srao.2003.50004. Semin Radiat Oncol. 2003. PMID: 12520462 Review.
-
Tumor angiogenesis and vascular normalization: alternative therapeutic targets.Angiogenesis. 2017 Nov;20(4):409-426. doi: 10.1007/s10456-017-9562-9. Epub 2017 Jun 28. Angiogenesis. 2017. PMID: 28660302 Review.
-
[Vascular perfusion as the origin of neoplasm resistance to radio- and chemotherapy].Rev Med Liege. 2010 Mar;65(3):133-9. Rev Med Liege. 2010. PMID: 20411817 French.
Cited by
-
Hyperthermia in Combination with Emerging Targeted and Immunotherapies as a New Approach in Cancer Treatment.Cancers (Basel). 2024 Jan 24;16(3):505. doi: 10.3390/cancers16030505. Cancers (Basel). 2024. PMID: 38339258 Free PMC article. Review.
-
Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments.Pharmaceutics. 2024 Jan 26;16(2):179. doi: 10.3390/pharmaceutics16020179. Pharmaceutics. 2024. PMID: 38399240 Free PMC article. Review.
-
Cell Culture Characterization of Prooxidative Chain-Transfer Agents as Novel Cytostatic Drugs.Molecules. 2021 Nov 8;26(21):6743. doi: 10.3390/molecules26216743. Molecules. 2021. PMID: 34771157 Free PMC article.
-
Signaling in and out: long-noncoding RNAs in tumor hypoxia.J Biomed Sci. 2020 May 5;27(1):59. doi: 10.1186/s12929-020-00654-x. J Biomed Sci. 2020. PMID: 32370770 Free PMC article. Review.
-
Tracking and recording of intracellular oxygen concentration changes in cell organelles: preparation and function of azide-modified fluorescent probes.RSC Adv. 2024 Jun 18;14(27):19586-19591. doi: 10.1039/d4ra01625d. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38895527 Free PMC article.
References
-
- Schwarz G. Ueber Desensibilisierung gegen röntgen- und radiumstrahlen. Munchener Medizinische Wochenschrift 1909; 24: 1–2.
-
- Mottram JC. A factor of importance in the radio sensitivity of tumours. Br J Radiol 1936; 9: 606–14. doi: 10.1259/0007-1285-9-105-606 - DOI
-
- Scott OC. Some aspects of the effect of ionizing radiation on tumors in experimental animals. Adv Biol Med Phys 1958; 6: 121–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

