A stimulus-responsive, in situ-forming, nanoparticle-laden hydrogel for ocular drug delivery
- PMID: 29508159
- PMCID: PMC5937863
- DOI: 10.1007/s13346-018-0504-x
A stimulus-responsive, in situ-forming, nanoparticle-laden hydrogel for ocular drug delivery
Abstract
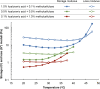
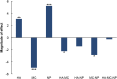
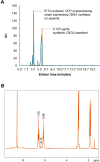
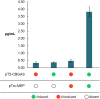
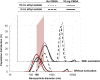
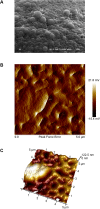
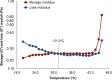
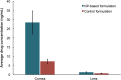
Most medications targeting optic neuropathies are administered as eye drops. However, their corneal penetration efficiencies are typically < 5%. There is a clear, unmet need for novel transcorneal drug delivery vehicles. To this end, we have developed a stimulus-responsive, in situ-forming, nanoparticle-laden hydrogel for controlled release of poorly bioavailable drugs into the aqueous humor of the eye. The hydrogel is formulated as a composite of hyaluronic acid (HA) and methylcellulose (MC). The amphiphilic nanoparticles are composed of poly(ethylene oxide) (PEO) and poly(lactic acid) (PLA). Experimental design aided the identification of hydrogel composition and nanoparticle content in the formulation, and the formulation reliably switched between thixotropy and temperature-dependent rheopexy when it was tested in a rheometer under conditions that simulate the ocular surface, including blinking. These properties should ensure that the formulation coats the cornea through blinking of the eyelid and facilitate application of the medication as an eye drop immediately prior to the patient's bedtime. We subsequently tested the efficacy of our formulation in whole-eye experiments by loading the nanoparticles with cannabigerolic acid (CBGA). Our formulation exhibits over a 300% increase in transcorneal penetration over control formulations. This work paves the way for the introduction of novel products targeting ocular diseases to the market.
Keywords: Cannabinoids; Glaucoma; Hydrogel; Nanoparticles; Switchable rheology; Synthetic biology.
Conflict of interest statement
Sazzad Hossain is the Chief Scientific Officer of InMed Pharmaceuticals Inc., a biopharmaceutical company that is commercializing an anti-glaucoma treatment that employs the formulation described in this study.
Figures

Similar articles
-
Biomineralized biomimetic organic/inorganic hybrid hydrogels based on hyaluronic acid and poloxamer.Carbohydr Polym. 2015 Aug 1;126:130-40. doi: 10.1016/j.carbpol.2015.03.033. Epub 2015 Mar 23. Carbohydr Polym. 2015. PMID: 25933531
-
Nanoparticle-based topical ophthalmic formulation for sustained release of stereoisomeric dipeptide prodrugs of ganciclovir.Drug Deliv. 2016 Sep;23(7):2399-2409. doi: 10.3109/10717544.2014.996833. Epub 2015 Jan 7. Drug Deliv. 2016. PMID: 25564964
-
Prevention of corneal neovascularization by subconjunctival injection of avastin® loaded thermosensitive hydrogels in rabbit model.Int J Pharm. 2018 Dec 1;552(1-2):164-170. doi: 10.1016/j.ijpharm.2018.09.017. Epub 2018 Sep 11. Int J Pharm. 2018. PMID: 30217769
-
Poly(lactic acid) based hydrogels.Adv Drug Deliv Rev. 2016 Dec 15;107:192-205. doi: 10.1016/j.addr.2016.07.004. Epub 2016 Jul 16. Adv Drug Deliv Rev. 2016. PMID: 27432797 Review.
-
Hyaluronic acid in ocular drug delivery.Carbohydr Polym. 2021 Jul 15;264:118006. doi: 10.1016/j.carbpol.2021.118006. Epub 2021 Mar 29. Carbohydr Polym. 2021. PMID: 33910737 Review.
Cited by
-
Current Advances in Nano-Based and Polymeric Stimuli-Responsive Drug Delivery Targeting the Ocular Microenvironment: A Review and Envisaged Future Perspectives.Polymers (Basel). 2022 Aug 30;14(17):3580. doi: 10.3390/polym14173580. Polymers (Basel). 2022. PMID: 36080651 Free PMC article. Review.
-
Multitarget, multiagent PLGA nanoparticles for simultaneous tumor eradication and TME remodeling in a melanoma mouse model.Drug Deliv Transl Res. 2024 Feb;14(2):491-509. doi: 10.1007/s13346-023-01413-9. Epub 2023 Aug 23. Drug Deliv Transl Res. 2024. PMID: 37612575 Free PMC article.
-
Cannabinoid-Based Ocular Therapies and Formulations.Pharmaceutics. 2023 Mar 27;15(4):1077. doi: 10.3390/pharmaceutics15041077. Pharmaceutics. 2023. PMID: 37111563 Free PMC article. Review.
-
Nanoparticle-Hydrogel Composite Drug Delivery System for Potential Ocular Applications.Polymers (Basel). 2021 Feb 21;13(4):642. doi: 10.3390/polym13040642. Polymers (Basel). 2021. PMID: 33670014 Free PMC article.
-
Active control of properties of concrete: a (p)review.Mater Struct. 2018;51(5):123. doi: 10.1617/s11527-018-1256-2. Epub 2018 Sep 20. Mater Struct. 2018. PMID: 30393457 Free PMC article.
References
-
- Riordan-Eva P. Anatomy & embryology of the eye. In: Riordan-Eva P, Cunningham ET, editors. Gen Ophthalmol. 18. New York, NY: McGraw-Hill; 1999. pp. 1–26.
-
- Optic neuropathy—pipeline review. London, UK; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

