Neonatal Apex Resection Triggers Cardiomyocyte Proliferation, Neovascularization and Functional Recovery Despite Local Fibrosis
- PMID: 29503089
- PMCID: PMC5918841
- DOI: 10.1016/j.stemcr.2018.01.042
Neonatal Apex Resection Triggers Cardiomyocyte Proliferation, Neovascularization and Functional Recovery Despite Local Fibrosis
Abstract
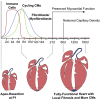
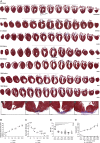
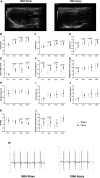
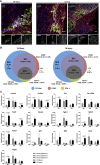
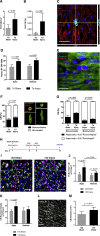
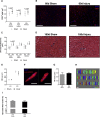
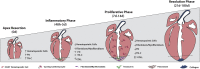
So far, opposing outcomes have been reported following neonatal apex resection in mice, questioning the validity of this injury model to investigate regenerative mechanisms. We performed a systematic evaluation, up to 180 days after surgery, of the pathophysiological events activated upon apex resection. In response to cardiac injury, we observed increased cardiomyocyte proliferation in remote and apex regions, neovascularization, and local fibrosis. In adulthood, resected hearts remain consistently shorter and display permanent fibrotic tissue deposition in the center of the resection plane, indicating limited apex regrowth. However, thickening of the left ventricle wall, explained by an upsurge in cardiomyocyte proliferation during the initial response to injury, compensated cardiomyocyte loss and supported normal systolic function. Thus, apex resection triggers both regenerative and reparative mechanisms, endorsing this injury model for studies aimed at promoting cardiomyocyte proliferation and/or downplaying fibrosis.
Keywords: cardiac fibroblasts; cardiac injury response; cardiac regeneration; cardiomyocyte proliferation; extracellular matrix; fibrosis; neonatal apex resection; neovascularization; stereology.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
A systematic analysis of neonatal mouse heart regeneration after apical resection.J Mol Cell Cardiol. 2015 Feb;79:315-8. doi: 10.1016/j.yjmcc.2014.12.011. Epub 2014 Dec 19. J Mol Cell Cardiol. 2015. PMID: 25533939 Free PMC article.
-
Persistent scarring and dilated cardiomyopathy suggest incomplete regeneration of the apex resected neonatal mouse myocardium--A 180 days follow up study.J Mol Cell Cardiol. 2016 Jan;90:47-52. doi: 10.1016/j.yjmcc.2015.11.031. Epub 2015 Nov 30. J Mol Cell Cardiol. 2016. PMID: 26655949
-
Regenerative responses after mild heart injuries for cardiomyocyte proliferation in zebrafish.Dev Dyn. 2014 Nov;243(11):1477-86. doi: 10.1002/dvdy.24171. Epub 2014 Aug 19. Dev Dyn. 2014. PMID: 25074230 Free PMC article.
-
Mechanisms of Neonatal Heart Regeneration.Curr Cardiol Rep. 2020 Apr 24;22(5):33. doi: 10.1007/s11886-020-01282-5. Curr Cardiol Rep. 2020. PMID: 32333123 Review.
-
Mechanisms of Cardiac Regeneration.Dev Cell. 2016 Feb 22;36(4):362-74. doi: 10.1016/j.devcel.2016.01.018. Dev Cell. 2016. PMID: 26906733 Free PMC article. Review.
Cited by
-
Development of a Bmi1+ Cardiac Mouse Progenitor Immortalized Model to Unravel the Relationship with Its Protective Vascular Endothelial Niche.Int J Mol Sci. 2024 Aug 13;25(16):8815. doi: 10.3390/ijms25168815. Int J Mol Sci. 2024. PMID: 39201501 Free PMC article.
-
Accelerated Growth, Differentiation, and Ploidy with Reduced Proliferation of Right Ventricular Cardiomyocytes in Children with Congenital Heart Defect Tetralogy of Fallot.Cells. 2022 Jan 5;11(1):175. doi: 10.3390/cells11010175. Cells. 2022. PMID: 35011735 Free PMC article.
-
Extracellular Matrix-Based Approaches in Cardiac Regeneration: Challenges and Opportunities.Int J Mol Sci. 2022 Dec 13;23(24):15783. doi: 10.3390/ijms232415783. Int J Mol Sci. 2022. PMID: 36555424 Free PMC article. Review.
-
Adult Cardiomyocyte Cell Cycle Detour: Off-ramp to Quiescent Destinations.Trends Endocrinol Metab. 2019 Aug;30(8):557-567. doi: 10.1016/j.tem.2019.05.006. Epub 2019 Jun 28. Trends Endocrinol Metab. 2019. PMID: 31262545 Free PMC article. Review.
-
Consistent Long-Term Therapeutic Efficacy of Human Umbilical Cord Matrix-Derived Mesenchymal Stromal Cells After Myocardial Infarction Despite Individual Differences and Transient Engraftment.Front Cell Dev Biol. 2021 Feb 4;9:624601. doi: 10.3389/fcell.2021.624601. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33614654 Free PMC article.
References
-
- Alkass K., Panula J., Westman M., Wu T.D., Guerquin-Kern J.L., Bergmann O. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell. 2015;163:1026–1036. - PubMed
-
- Andersen D.C., Jensen C.H., Baun C., Hvidsten S., Zebrowski D.C., Engel F.B., Sheikh S.P. Persistent scarring and dilated cardiomyopathy suggest incomplete regeneration of the apex resected neonatal mouse myocardium – a 180 days follow up study. J. Mol. Cell. Cardiol. 2016;90:47–52. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

