Functional Characterization of ABCC Proteins from Trypanosoma cruzi and Their Involvement with Thiol Transport
- PMID: 29491856
- PMCID: PMC5817095
- DOI: 10.3389/fmicb.2018.00205
Functional Characterization of ABCC Proteins from Trypanosoma cruzi and Their Involvement with Thiol Transport
Abstract
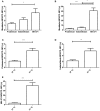
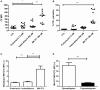
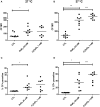
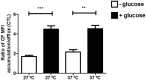
Chagas disease is a neglected disease caused by the protozoan Trypanosoma cruzi and affects 8 million people worldwide. The main chemotherapy is based on benznidazole. The efficacy in the treatment depends on factors such as the parasite strain, which may present different sensitivity to treatment. In this context, the expression of ABC transporters has been related to chemotherapy failure. ABC transporters share a well-conserved ABC domain, responsible for ATP binding and hydrolysis, whose the energy released is coupled to transport of molecules through membranes. The most known ABC transporters are ABCB1 and ABCC1, involved in the multidrug resistance phenotype in cancer, given their participation in cellular detoxification. In T. cruzi, 27 ABC genes were identified in the genome. Nonetheless, only four ABC genes were characterized: ABCA3, involved in vesicular trafficking; ABCG1, overexpressed in strains naturally resistant to benznidazole, and P-glycoprotein 1 and 2, whose participation in drug resistance is controversial. Considering P-glycoprotein genes are related to ABCC subfamily in T. cruzi according to the demonstration using BLASTP alignment, we evaluated both ABCB1-like and ABCC-like activities in epimastigote and trypomastigote forms of the Y strain. The transport activities were evaluated by the efflux of the fluorescent dyes Rhodamine 123 and Carboxyfluorescein in a flow cytometer. Results indicated that there was no ABCB1-like activity in both T. cruzi forms. Conversely, results demonstrated ABCC-like activity in both epimastigote and trypomastigote forms of T. cruzi. This activity was inhibited by ABCC transport modulators (probenecid, indomethacin, and MK-571), by ATP-depleting agents (sodium azide and iodoacetic acid) and by the thiol-depleting agent N-ethylmaleimide. Additionally, the presence of ABCC-like activity was supported by direct inhibition of the thiol-conjugated compound efflux with indomethacin, characteristic of ABCC subfamily members. Taken together, the results provide the first description of native ABCC-like activity in T. cruzi epimastigote and trypomastigote forms, indicating that the study of the biological role for that thiol transporter is crucial to reveal new molecular mechanisms for therapeutic approaches in the Chagas disease.
Keywords: ABC transporters; Chagas disease; P-glycoprotein; Trypanosoma cruzi; multidrug resistance phenotype; multidrug resistance protein; thiol transporter.
Figures



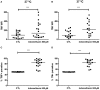
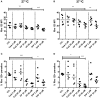
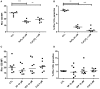
Similar articles
-
The History of the ABC Proteins in Human Trypanosomiasis Pathogens.Pathogens. 2022 Aug 30;11(9):988. doi: 10.3390/pathogens11090988. Pathogens. 2022. PMID: 36145420 Free PMC article. Review.
-
Intrinsic and Chemotherapeutic Stressors Modulate ABCC-Like Transport in Trypanosoma cruzi.Molecules. 2021 Jun 9;26(12):3510. doi: 10.3390/molecules26123510. Molecules. 2021. PMID: 34207619 Free PMC article.
-
The multi-xenobiotic resistance (MXR) efflux activity in hemocytes of Mytilus edulis is mediated by an ATP binding cassette transporter of class C (ABCC) principally inducible in eosinophilic granulocytes.Aquat Toxicol. 2014 Aug;153:98-109. doi: 10.1016/j.aquatox.2013.11.012. Epub 2013 Nov 28. Aquat Toxicol. 2014. PMID: 24345773
-
Abcb and Abcc transporter homologs are expressed and active in larvae and adults of zebra mussel and induced by chemical stress.Aquat Toxicol. 2012 Oct 15;122-123:144-52. doi: 10.1016/j.aquatox.2012.06.008. Epub 2012 Jun 28. Aquat Toxicol. 2012. PMID: 22819804
-
Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development.Curr Med Chem. 2008;15(20):1981-2039. doi: 10.2174/092986708785132870. Curr Med Chem. 2008. PMID: 18691054 Review.
Cited by
-
Genomic Insight of Leishmania Parasite: In-Depth Review of Drug Resistance Mechanisms and Genetic Mutations.ACS Omega. 2024 Mar 8;9(11):12500-12514. doi: 10.1021/acsomega.3c09400. eCollection 2024 Mar 19. ACS Omega. 2024. PMID: 38524425 Free PMC article. Review.
-
Graphene quantum dots induce cascadic apoptosis via interaction with proteins associated with anti-oxidation after endocytosis by Trypanosoma brucei.Front Immunol. 2022 Dec 6;13:1022050. doi: 10.3389/fimmu.2022.1022050. eCollection 2022. Front Immunol. 2022. PMID: 36561761 Free PMC article.
-
The History of the ABC Proteins in Human Trypanosomiasis Pathogens.Pathogens. 2022 Aug 30;11(9):988. doi: 10.3390/pathogens11090988. Pathogens. 2022. PMID: 36145420 Free PMC article. Review.
-
The repositioned drugs disulfiram/diethyldithiocarbamate combined to benznidazole: Searching for Chagas disease selective therapy, preventing toxicity and drug resistance.Front Cell Infect Microbiol. 2022 Jul 29;12:926699. doi: 10.3389/fcimb.2022.926699. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35967878 Free PMC article.
-
Intrinsic and Chemotherapeutic Stressors Modulate ABCC-Like Transport in Trypanosoma cruzi.Molecules. 2021 Jun 9;26(12):3510. doi: 10.3390/molecules26123510. Molecules. 2021. PMID: 34207619 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

