PGRP-LD mediates A. stephensi vector competency by regulating homeostasis of microbiota-induced peritrophic matrix synthesis
- PMID: 29489896
- PMCID: PMC5831637
- DOI: 10.1371/journal.ppat.1006899
PGRP-LD mediates A. stephensi vector competency by regulating homeostasis of microbiota-induced peritrophic matrix synthesis
Abstract
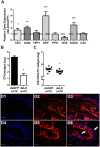
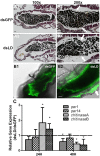
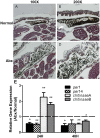
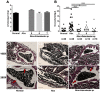
Peptidoglycan recognition proteins (PGRPs) and commensal microbes mediate pathogen infection outcomes in insect disease vectors. Although PGRP-LD is retained in multiple vectors, its role in host defense remains elusive. Here we report that Anopheles stephensi PGRP-LD protects the vector from malaria parasite infection by regulating gut homeostasis. Specifically, knock down of PGRP-LD (dsLD) increased susceptibility to Plasmodium berghei infection, decreased the abundance of gut microbiota and changed their spatial distribution. This outcome resulted from a change in the structural integrity of the peritrophic matrix (PM), which is a chitinous and proteinaceous barrier that lines the midgut lumen. Reduction of microbiota in dsLD mosquitoes due to the upregulation of immune effectors led to dysregulation of PM genes and PM fragmentation. Elimination of gut microbiota in antibiotic treated mosquitoes (Abx) led to PM loss and increased vectorial competence. Recolonization of Abx mosquitoes with indigenous Enterobacter sp. restored PM integrity and decreased mosquito vectorial capacity. Silencing PGRP-LD in mosquitoes without PM didn't influence their vector competence. Our results indicate that PGPR-LD protects the gut microbiota by preventing hyper-immunity, which in turn promotes PM structurally integrity. The intact PM plays a key role in limiting P. berghei infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Gut microbiota is essential in PGRP-LA regulated immune protection against Plasmodium berghei infection.Parasit Vectors. 2020 Jan 6;13(1):3. doi: 10.1186/s13071-019-3876-y. Parasit Vectors. 2020. PMID: 31907025 Free PMC article.
-
Influence of midgut microbiota in Anopheles stephensi on Plasmodium berghei infections.Malar J. 2018 Oct 25;17(1):385. doi: 10.1186/s12936-018-2535-7. Malar J. 2018. PMID: 30359252 Free PMC article.
-
Phenotypic dissection of a Plasmodium-refractory strain of malaria vector Anopheles stephensi: the reduced susceptibility to P. berghei and P. yoelii.PLoS One. 2013 May 23;8(5):e63753. doi: 10.1371/journal.pone.0063753. Print 2013. PLoS One. 2013. PMID: 23717475 Free PMC article.
-
The tripartite interactions between the mosquito, its microbiota and Plasmodium.Parasit Vectors. 2018 Mar 20;11(1):200. doi: 10.1186/s13071-018-2784-x. Parasit Vectors. 2018. PMID: 29558973 Free PMC article. Review.
-
Interrupting malaria transmission by genetic manipulation of anopheline mosquitoes.J Vector Borne Dis. 2003 Sep-Dec;40(3-4):73-7. J Vector Borne Dis. 2003. PMID: 15119075 Review.
Cited by
-
The microbiome and mosquito vectorial capacity: rich potential for discovery and translation.Microbiome. 2021 May 18;9(1):111. doi: 10.1186/s40168-021-01073-2. Microbiome. 2021. PMID: 34006334 Free PMC article. Review.
-
C-type lectin-mediated microbial homeostasis is critical for Helicoverpa armigera larval growth and development.PLoS Pathog. 2020 Sep 30;16(9):e1008901. doi: 10.1371/journal.ppat.1008901. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32997722 Free PMC article.
-
Mosquito Trilogy: Microbiota, Immunity and Pathogens, and Their Implications for the Control of Disease Transmission.Front Microbiol. 2021 Apr 6;12:630438. doi: 10.3389/fmicb.2021.630438. eCollection 2021. Front Microbiol. 2021. PMID: 33889137 Free PMC article. Review.
-
Second generation effects of larval metal pollutant exposure on reproduction, longevity and insecticide tolerance in the major malaria vector Anopheles arabiensis (Diptera: Culicidae).Parasit Vectors. 2020 Jan 7;13(1):4. doi: 10.1186/s13071-020-3886-9. Parasit Vectors. 2020. PMID: 31910892 Free PMC article.
-
Glucose transporter GLUT1 influences Plasmodium berghei infection in Anopheles stephensi.Parasit Vectors. 2020 Jun 5;13(1):285. doi: 10.1186/s13071-020-04155-6. Parasit Vectors. 2020. PMID: 32503601 Free PMC article.
References
-
- World Health Organization (2015) World Malaria Report 2015.
-
- Wang S, Jacobs-Lorena M (2013) Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol 31: 185–193. doi: 10.1016/j.tibtech.2013.01.001 - DOI - PMC - PubMed
-
- Clayton AM, Dong Y, Dimopoulos G (2014) The Anopheles innate immune system in the defense against malaria infection. J Innate Immun 6: 169–181. doi: 10.1159/000353602 - DOI - PMC - PubMed
-
- Marois E (2011) The multifaceted mosquito anti-Plasmodium response. Curr Opin Microbiol 14: 429–435. doi: 10.1016/j.mib.2011.07.016 - DOI - PubMed
-
- Hegedus D, Erlandson M, Gillott C, Toprak U (2009) New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol 54: 285–302. doi: 10.1146/annurev.ento.54.110807.090559 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

