The multi-receptor inhibitor axitinib reverses tumor-induced immunosuppression and potentiates treatment with immune-modulatory antibodies in preclinical murine models
- PMID: 29487979
- PMCID: PMC11028099
- DOI: 10.1007/s00262-018-2136-x
The multi-receptor inhibitor axitinib reverses tumor-induced immunosuppression and potentiates treatment with immune-modulatory antibodies in preclinical murine models
Abstract
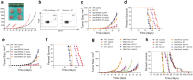
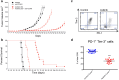
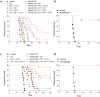
Cancer immunotherapies have significantly improved the prognosis of cancer patients. Despite the clinical success of targeting inhibitory checkpoint receptors, including PD-1 and/or CTLA-4 on T cells, only a minority of patients derive benefit from these therapies. New strategies to improve cancer immunotherapy are therefore needed. Combination therapy of checkpoint inhibitors with targeted agents has promisingly shown to increase the efficacy of immunotherapy. Here, we analyzed the immunomodulatory effects of the multi-receptor tyrosine kinase inhibitor axitinib and its efficacy in combination with immunotherapies. In different syngeneic murine tumor models, axitinib showed therapeutic efficacy that was not only mediated by VEGF-VEGFR inhibition, but also through the induction of anti-cancer immunity. Mechanistically, a significant reduction of immune-suppressive cells, including a decrease of tumor-promoting mast cells and tumor-associated macrophages was observed upon axitinib treatment. Inhibition of mast cells by axitinib as well as their experimental depletion led to reduced tumor growth. Of note, treatment with axitinib led to an improved T cell response, while the latter was pivotal for the therapeutic efficacy. Combination with immune checkpoint inhibitors anti-PD-1 and anti-TIM-3 and/or agonistic engagement of the activating receptor CD137 resulted in a synergistic therapeutic efficacy. This demonstrates non-redundant immune activation induced by axitinib via modulation of myeloid and mast cells. These findings provide important mechanistic insights into axitinib-mediated anti-cancer immunity and provide rationale for clinical combinations of axitinib with different immunotherapeutic modalities.
Keywords: Cancer immunology; Hematopoietic stem cell; Mast cell; Tumor-associated macrophage; Tyrosine kinase inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3.Clin Cancer Res. 2008 Nov 15;14(22):7272-83. doi: 10.1158/1078-0432.CCR-08-0652. Clin Cancer Res. 2008. PMID: 19010843
-
Disease progression in recurrent glioblastoma patients treated with the VEGFR inhibitor axitinib is associated with increased regulatory T cell numbers and T cell exhaustion.Cancer Immunol Immunother. 2016 Jun;65(6):727-40. doi: 10.1007/s00262-016-1836-3. Epub 2016 Apr 20. Cancer Immunol Immunother. 2016. PMID: 27098427 Free PMC article. Clinical Trial.
-
Folate-Hapten-Mediated Immunotherapy Synergizes with Vascular Endothelial Growth Factor Receptor Inhibitors in Treating Murine Models of Cancer.Mol Cancer Ther. 2017 Mar;16(3):461-468. doi: 10.1158/1535-7163.MCT-16-0569. Epub 2016 Dec 15. Mol Cancer Ther. 2017. PMID: 27980109 Free PMC article.
-
Axitinib in metastatic renal cell carcinoma.Expert Rev Anticancer Ther. 2015 May;15(5):499-507. doi: 10.1586/14737140.2015.1033408. Expert Rev Anticancer Ther. 2015. PMID: 25907705 Review.
-
Axitinib: from preclinical development to future clinical perspectives in renal cell carcinoma.Expert Opin Drug Discov. 2015;10(8):925-35. doi: 10.1517/17460441.2015.1045411. Epub 2015 Jun 3. Expert Opin Drug Discov. 2015. PMID: 26039031 Review.
Cited by
-
Enhancing the Potential of Immunotherapy in Paediatric Sarcomas: Breaking the Immunosuppressive Barrier with Receptor Tyrosine Kinase Inhibitors.Biomedicines. 2021 Nov 30;9(12):1798. doi: 10.3390/biomedicines9121798. Biomedicines. 2021. PMID: 34944614 Free PMC article. Review.
-
Targeting Multiple Receptors to Increase Checkpoint Blockade Efficacy.Int J Mol Sci. 2019 Jan 4;20(1):158. doi: 10.3390/ijms20010158. Int J Mol Sci. 2019. PMID: 30621125 Free PMC article. Review.
-
Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review.Int J Cancer. 2019 Sep 1;145(5):1179-1188. doi: 10.1002/ijc.32020. Epub 2019 Jan 7. Int J Cancer. 2019. PMID: 30478914 Free PMC article. Review.
-
Oncolytic Virus Encoding a Master Pro-Inflammatory Cytokine Interleukin 12 in Cancer Immunotherapy.Cells. 2020 Feb 10;9(2):400. doi: 10.3390/cells9020400. Cells. 2020. PMID: 32050597 Free PMC article. Review.
-
Co-stimulatory agonists: An insight into the immunotherapy of cancer.EXCLI J. 2021 Jun 9;20:1055-1085. doi: 10.17179/excli2021-3522. eCollection 2021. EXCLI J. 2021. PMID: 34267616 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

