Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies
- PMID: 29485986
- PMCID: PMC5828349
- DOI: 10.1371/journal.pmed.1002511
Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies
Erratum in
-
Correction: Prevalence of sexually transmitted infections and bacterial vaginosis among women in sub-Saharan Africa: An individual participant data meta-analysis of 18 HIV prevention studies.PLoS Med. 2018 Jun 26;15(6):e1002608. doi: 10.1371/journal.pmed.1002608. eCollection 2018 Jun. PLoS Med. 2018. PMID: 29944660 Free PMC article.
Abstract
Background: Estimates of sexually transmitted infection (STI) prevalence are essential for efforts to prevent and control STIs. Few large STI prevalence studies exist, especially for low- and middle-income countries (LMICs). Our primary objective was to estimate the prevalence of chlamydia, gonorrhea, trichomoniasis, syphilis, herpes simplex virus type 2 (HSV-2), and bacterial vaginosis (BV) among women in sub-Saharan Africa by age, region, and population type.
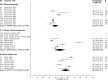
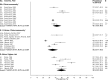
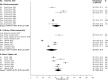
Methods and findings: We analyzed individual-level data from 18 HIV prevention studies (cohort studies and randomized controlled trials; conducted during 1993-2011), representing >37,000 women, that tested participants for ≥1 selected STIs or BV at baseline. We used a 2-stage meta-analysis to combine data. After calculating the proportion of participants with each infection and standard error by study, we used a random-effects model to obtain a summary mean prevalence of each infection and 95% confidence interval (CI) across ages, regions, and population types. Despite substantial study heterogeneity for some STIs/populations, several patterns emerged. Across the three primary region/population groups (South Africa community-based, Southern/Eastern Africa community-based, and Eastern Africa higher-risk), prevalence was higher among 15-24-year-old than 25-49-year-old women for all STIs except HSV-2. In general, higher-risk populations had greater prevalence of gonorrhea and syphilis than clinic/community-based populations. For chlamydia, prevalence among 15-24-year-olds was 10.3% (95% CI: 7.4%, 14.1%; I2 = 75.7%) among women specifically recruited from higher-risk settings for HIV in Eastern Africa and was 15.1% (95% CI: 12.7%, 17.8%; I2 = 82.3%) in South African clinic/community-based populations. Among clinic/community-based populations, prevalence was generally greater in South Africa than in Southern/Eastern Africa for most STIs; for gonorrhea, prevalence among 15-24-year-olds was 4.6% (95% CI: 3.3%, 6.4%; I2 = 82.8%) in South Africa and was 1.7% (95% CI: 1.2%, 2.6%; I2 = 55.2%) in Southern/Eastern Africa. Across the three primary region/population groups, HSV-2 and BV prevalence was high among 25-49-year-olds (ranging from 70% to 83% and 33% to 44%, respectively). The main study limitation is that the data are not from random samples of the target populations.
Conclusions: Combining data from 18 HIV prevention studies, our findings highlight important features of STI/BV epidemiology among sub-Saharan African women. This methodology can be used where routine STI surveillance is limited and offers a new approach to obtaining critical information on STI and BV prevalence in LMICs.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: RH receives royalties for textbook from Chapman and Hall and occasional attendance fees for serving on the MRC funding committee. KJL received funding from the World Health Organization during the course of this study. NL receives a stipend as a Specialty Consulting Editor for
Figures











Similar articles
-
Prevalence of curable STIs and bacterial vaginosis during pregnancy in sub-Saharan Africa: a systematic review and meta-analysis.Sex Transm Infect. 2022 Nov;98(7):484-491. doi: 10.1136/sextrans-2021-055057. Epub 2021 Dec 9. Sex Transm Infect. 2022. PMID: 34887350 Free PMC article.
-
Molecular Identification of Cervical Microbes in HIV-Negative and HIV-Positive Women in an African Setting Using a Customized Bacterial Vaginosis Microbial DNA Quantitative PCR (qPCR) Array.Microbiol Spectr. 2022 Jun 29;10(3):e0222921. doi: 10.1128/spectrum.02229-21. Epub 2022 Jun 1. Microbiol Spectr. 2022. PMID: 35647888 Free PMC article.
-
Sexually transmitted infections in young pregnant women in Bangui, Central African Republic.Int J STD AIDS. 1999 Sep;10(9):609-14. doi: 10.1258/0956462991914753. Int J STD AIDS. 1999. PMID: 10492429
-
Structural and community-level interventions for increasing condom use to prevent the transmission of HIV and other sexually transmitted infections.Cochrane Database Syst Rev. 2014 Jul 29;2014(7):CD003363. doi: 10.1002/14651858.CD003363.pub3. Cochrane Database Syst Rev. 2014. PMID: 25072817 Free PMC article. Review.
-
Population-based biomedical sexually transmitted infection control interventions for reducing HIV infection.Cochrane Database Syst Rev. 2011 Mar 16;(3):CD001220. doi: 10.1002/14651858.CD001220.pub3. Cochrane Database Syst Rev. 2011. PMID: 21412869 Review.
Cited by
-
Understanding the preferences of young women in self-sampling interventions for sexually transmitted infection diagnosis: a discrete choice experimental protocol.BMJ Open. 2024 Sep 24;14(9):e082981. doi: 10.1136/bmjopen-2023-082981. BMJ Open. 2024. PMID: 39317498 Free PMC article.
-
Determinants of care-seeking behavior for sexually transmitted infections among sexually active men in East Africa: A multilevel mixed effect analysis.PLoS One. 2024 Sep 5;19(9):e0307755. doi: 10.1371/journal.pone.0307755. eCollection 2024. PLoS One. 2024. PMID: 39236062 Free PMC article.
-
Prevalence of sexually transmitted infections, and its associated factors among students in Ethiopia: a systematic review and meta-analysis study.BMC Public Health. 2024 Jul 24;24(1):1976. doi: 10.1186/s12889-024-19548-w. BMC Public Health. 2024. PMID: 39049035 Free PMC article.
-
Bacterial vaginosis-driven changes in vaginal T cell phenotypes and their implications for HIV susceptibility.bioRxiv [Preprint]. 2024 Jul 5:2024.07.03.601916. doi: 10.1101/2024.07.03.601916. bioRxiv. 2024. PMID: 39005354 Free PMC article. Preprint.
-
High STI burden among a cohort of adolescents aged 12-19 years in a youth-friendly clinic in South Africa.PLoS One. 2024 Jul 10;19(7):e0306771. doi: 10.1371/journal.pone.0306771. eCollection 2024. PLoS One. 2024. PMID: 38985722 Free PMC article.
References
-
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, et al. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS ONE. 2015;10(12):e0143304 doi: 10.1371/journal.pone.0143304 - DOI - PMC - PubMed
-
- Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 2015;10(1):e114989 doi: 10.1371/journal.pone.0114989 - DOI - PMC - PubMed
-
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209(6):505–23. doi: 10.1016/j.ajog.2013.05.006 - DOI - PubMed
-
- World Health Organization. Global health sector strategy on sexually transmitted infections 2016–2021: towards ending STIs. Geneva: World Health Organization; 2016. July [cited 2017 Aug 3]. Available from: http://apps.who.int/iris/bitstream/10665/246296/1/WHO-RHR-16.09-eng.pdf?....
-
- World Health Organization. Meeting report: consultation on methods for improved global sexually transmitted infections estimates November 11–12, 2013, Geneva, Switzerland. Geneva: World Health Organization; 2014. March.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

