Coroglaucigenin induces senescence and autophagy in colorectal cancer cells
- PMID: 29484762
- PMCID: PMC6528924
- DOI: 10.1111/cpr.12451
Coroglaucigenin induces senescence and autophagy in colorectal cancer cells
Abstract
Objectives: Coroglaucigenin (CGN), a natural product isolated from Calotropis gigantean by our research group, has been identified as a potential anti-cancer agent. However, the molecular mechanisms involved remain poorly understood.
Materials and methods: Cell viability and cell proliferation were detected by MTT and BrdU assays. Flow cytometry, SA-β-gal assay, western blotting and immunofluorescence were performed to determine CGN-induced apoptosis, senescence and autophagy. Western blotting, siRNA transfection and coimmunoprecipitation were carried out to investigate the mechanisms of CGN-induced senescence and autophagy. The anti-tumour activities of combination therapy with CGN and chloroquine were observed in mice tumour models.
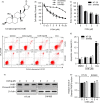
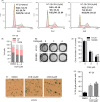
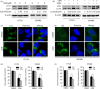
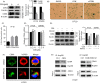
Results: We demonstrated that CGN inhibits the proliferation of colorectal cancer cells both in vitro and in vivo. We showed that the inhibition of cell proliferation by CGN is independent of apoptosis, but is associated with cell-cycle arrest and senescence in colorectal cancer cells. Notably, CGN induces protective autophagy that attenuates CGN-mediated cell proliferation. Functional studies revealed that CGN disrupts the association of Hsp90 with both CDK4 and Akt, leading to CDK4 degradation and Akt dephosphorylation, eventually resulting in senescence and autophagy, respectively. Combination therapy with CGN and chloroquine resulted in enhanced anti-tumour effects in vivo.
Conclusions: Our results demonstrate that CGN induces senescence and autophagy in colorectal cancer cells and indicate that combining it with an autophagy inhibitor may be a novel strategy suitable for CGN-mediated anti-cancer therapy.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
There is no competing interest.
Figures
Similar articles
-
3'-epi-12β-hydroxyfroside, a new cardenolide, induces cytoprotective autophagy via blocking the Hsp90/Akt/mTOR axis in lung cancer cells.Theranostics. 2018 Feb 15;8(7):2044-2060. doi: 10.7150/thno.23304. eCollection 2018. Theranostics. 2018. PMID: 29556372 Free PMC article.
-
Licoricidin inhibits the growth of SW480 human colorectal adenocarcinoma cells in vitro and in vivo by inducing cycle arrest, apoptosis and autophagy.Toxicol Appl Pharmacol. 2017 Jul 1;326:25-33. doi: 10.1016/j.taap.2017.04.015. Epub 2017 Apr 14. Toxicol Appl Pharmacol. 2017. PMID: 28416456
-
Inhibitory Effect of Alnustone on Survival and Lung Metastasis of Colorectal Cancer Cells.Nutrients. 2024 Oct 31;16(21):3737. doi: 10.3390/nu16213737. Nutrients. 2024. PMID: 39519569 Free PMC article.
-
CDK4/6 Inhibitors: The Mechanism of Action May Not Be as Simple as Once Thought.Cancer Cell. 2018 Jul 9;34(1):9-20. doi: 10.1016/j.ccell.2018.03.023. Epub 2018 May 3. Cancer Cell. 2018. PMID: 29731395 Free PMC article. Review.
-
Autophagy: Mechanisms and Therapeutic Potential of Flavonoids in Cancer.Biomolecules. 2021 Jan 21;11(2):135. doi: 10.3390/biom11020135. Biomolecules. 2021. PMID: 33494431 Free PMC article. Review.
Cited by
-
An updated pharmacological insight into calotropin as a potential therapeutic agent in cancer.Front Pharmacol. 2023 Apr 17;14:1160616. doi: 10.3389/fphar.2023.1160616. eCollection 2023. Front Pharmacol. 2023. PMID: 37138852 Free PMC article. Review.
-
Calotropis gigantea stem bark extract induced apoptosis related to ROS and ATP production in colon cancer cells.PLoS One. 2021 Aug 3;16(8):e0254392. doi: 10.1371/journal.pone.0254392. eCollection 2021. PLoS One. 2021. PMID: 34343190 Free PMC article.
-
Tapping the potential of Calotropis procera hairy roots for cardiac glycosides production and their identification using UHPLC/QTOF-MS.3 Biotech. 2024 Sep;14(9):199. doi: 10.1007/s13205-024-04035-1. Epub 2024 Aug 12. 3 Biotech. 2024. PMID: 39144068
-
Autophagy of macrophages is regulated by PI3k/Akt/mTOR signalling in the development of diabetic encephalopathy.Aging (Albany NY). 2018 Oct 22;10(10):2772-2782. doi: 10.18632/aging.101586. Aging (Albany NY). 2018. PMID: 30346929 Free PMC article.
-
The overexpression of Tipe2 in CRC cells suppresses survival while endogenous Tipe2 accelerates AOM/DSS induced-tumor initiation.Cell Death Dis. 2021 Oct 26;12(11):1001. doi: 10.1038/s41419-021-04289-0. Cell Death Dis. 2021. PMID: 34702807 Free PMC article.
References
-
- Gheorghiade M, van Veldhuisen DJ, Colucci WS. Contemporary use of digoxin in the management of cardiovascular disorders. Circulation. 2006;113:2556‐2564. - PubMed
-
- Newman RA, Yang P, Pawlus AD, et al. Cardiac glycosides as novel cancer therapeutic agents. Mol Interv. 2008;8:36‐49. - PubMed
-
- Cerella C, Dicato M, Diederich M. Assembling the puzzle of anti‐cancer mechanisms triggered by cardiac glycosides. Mitochondrion. 2013;13:225‐234. - PubMed
-
- McConkey DJ, Lin Y, Nutt LK, et al. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen‐independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000;60:3807‐3812. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

