Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment
- PMID: 29484379
- PMCID: PMC5846648
- DOI: 10.3892/ijmm.2018.3501
Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment
Abstract
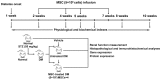
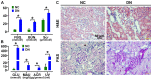
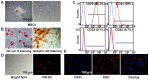
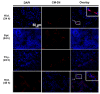
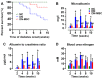
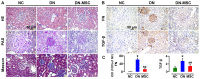
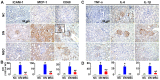
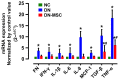
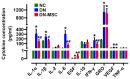
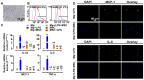
Diabetic nephropathy (DN) is a major complication of diabetes and represents the leading cause of end-stage renal disease. Mesenchymal stem cell (MSC) treatment has been demonstrated to be effective in DN models by reducing albuminuria and attenuating glomerular injury; however, limited in-depth understanding of the underlying mechanism and a lack of clinical trials hinders its clinical use. Additionally, most of these experimental studies were conducted on the advanced stage of nephropathy, which is difficult to reverse and consequently showed limited therapeutic efficacy. We sought to evaluate whether early intervention by MSCs has the potential to prevent DN onset and progression as well as protect kidney function when intravenously administered to rats with diabetes. Diabetes was induced in adult male SD rats by streptozotocin (STZ) injection (55 mg/kg, i.p.). The diabetic rats were injected with or without bone marrow-derived MSCs (5x106 per rat), via tail vein at 2, 4, 5 and 7 weeks after diabetes onset. Fasting blood glucose (FBG), blood urea nitrogen (BUN) and serum creatinine (Scr) levels in serum samples and glycosuria (GLU), microalbumin (MAU), and albumin to creatinine ratio (ACR) in urine samples were determined. Renal pathology and immunohistochemistry (IHC) for CD68, MCP-1, fibronectin (FN), transforming growth factor-β (TGF-β) and pro-inflammatory cytokines were also performed. Expression levels of the above factors as well as interleukin-10 (IL-10), and epidermal growth factor (EGF) were assessed by qPCR and multiplex bead-based suspension array system, respectively. Additionally, MSC tracing in vivo was performed. Ex vivo, peritoneal macrophages were co-cultured with MSCs, and expression of inflammatory cytokines was detected as well. MSC treatment profoundly suppressed renal macrophage infiltration and inflammatory cytokine secretion in diabetic rats, resulting in prominently improved kidney histology, systemic homeostasis, and animal survival, although no significant effect on hyperglycemia was observed. Engrafted MSCs were primarily localized in deteriorated areas of the kidney and immune organs 48 h after infusion. MSC treatment upregulated serum anti-inflammatory cytokines IL-10 and EGF. Ex vivo, MSCs inhibited lipopolysaccharide (LPS)-stimulated rat peritoneal macrophage activation via the downregulation of inflammatory-related cytokines such as IL-6, MCP-1, tumor necrosis factor-α (TNF-α) and IL-1β. Our results demonstrated that early intervention with MSCs prevented renal injury via immune regulation in diabetic rats, which restored the homeostasis of the immune microenvironment, contributing to the prevention of kidney dysfunction and glomerulosclerosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocin-induced diabetic nephropathy in rats via inhibiting macrophage infiltration.Int Immunopharmacol. 2013 Oct;17(2):275-82. doi: 10.1016/j.intimp.2013.05.031. Epub 2013 Jun 19. Int Immunopharmacol. 2013. PMID: 23791972
-
Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis.Stem Cell Res Ther. 2020 Aug 3;11(1):336. doi: 10.1186/s13287-020-01852-y. Stem Cell Res Ther. 2020. PMID: 32746936 Free PMC article.
-
Mesenchymal Stem Cells Reverse Diabetic Nephropathy Disease via Lipoxin A4 by Targeting Transforming Growth Factor β (TGF-β)/smad Pathway and Pro-Inflammatory Cytokines.Med Sci Monit. 2019 Apr 26;25:3069-3076. doi: 10.12659/MSM.914860. Med Sci Monit. 2019. PMID: 31023998 Free PMC article.
-
Cytokines in diabetic nephropathy.Adv Clin Chem. 2012;56:55-74. doi: 10.1016/b978-0-12-394317-0.00014-5. Adv Clin Chem. 2012. PMID: 22397028 Review.
-
The Multi-Therapeutic Role of MSCs in Diabetic Nephropathy.Front Endocrinol (Lausanne). 2021 Jun 7;12:671566. doi: 10.3389/fendo.2021.671566. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34163437 Free PMC article. Review.
Cited by
-
Sodium Butyrate Attenuates Diabetic Kidney Disease Partially via Histone Butyrylation Modification.Mediators Inflamm. 2022 Jul 20;2022:7643322. doi: 10.1155/2022/7643322. eCollection 2022. Mediators Inflamm. 2022. PMID: 35909658 Free PMC article.
-
Sacubitril/Valsartan Improves Progression of Early Diabetic Nephropathy in Rats Through Inhibition of NLRP3 Inflammasome Pathway.Diabetes Metab Syndr Obes. 2022 Aug 13;15:2479-2488. doi: 10.2147/DMSO.S366518. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 35992034 Free PMC article.
-
The Use of Pulsed Electromagnetic Field to Modulate Inflammation and Improve Tissue Regeneration: A Review.Bioelectricity. 2019 Dec 1;1(4):247-259. doi: 10.1089/bioe.2019.0026. Epub 2019 Dec 12. Bioelectricity. 2019. PMID: 34471827 Free PMC article. Review.
-
Glycemic control by umbilical cord-derived mesenchymal stem cells promotes effects of fasting-mimicking diet on type 2 diabetic mice.Stem Cell Res Ther. 2021 Jul 13;12(1):395. doi: 10.1186/s13287-021-02467-7. Stem Cell Res Ther. 2021. PMID: 34256832 Free PMC article.
-
Fibrosis in Chronic Kidney Disease: Pathophysiology and Therapeutic Targets.J Clin Med. 2024 Mar 25;13(7):1881. doi: 10.3390/jcm13071881. J Clin Med. 2024. PMID: 38610646 Free PMC article. Review.
References
-
- Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney APS, Briggs D, et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: Results from an international comparative study. Am J Kidney Dis. 2000;35:157–165. doi: 10.1016/S0272-6386(00)70316-7. - DOI - PubMed
-
- McCrary EB. The road to renal failure: An overview of diabetic nephropathy. Adv Nurse Pract. 2008;16:61–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

