Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis
- PMID: 29484119
- PMCID: PMC5800911
- DOI: 10.18632/oncotarget.23238
Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis
Abstract
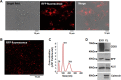
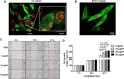
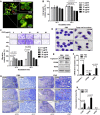
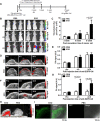
Crosstalk between breast cancer and macrophages has potential implications for tumor metastasis. This study investigates macrophage polarization induced by triple-negative breast cancer (TNBC) cell-derived exosomes that promote lymph node (LN) metastasis in orthotopic TNBC models. The MDA-MB-231 cancer cell line expressing the exosomal CD63-red fluorescence (RFP) fusion protein was generated to noninvasively visualize exosome transfer into cancer cells and macrophages. Administration of RFP-tagged exosomes enhanced migration of macrophages and induced macrophage polarization in vitro. In orthotopic TNBC models, noninvasive bioluminescent imaging, ultrasound-guided photoacoustic imaging, and histological analysis revealed that intravenous injection of RFP-tagged exosomes promoted primary tumor growth and axillary LN metastasis in which expression of CD206, a marker or alternatively activated type 2 (M2) macrophages, was significantly higher than expression of NOS2, a marker of classically activated type 1 (M1) macrophages. These results suggest breast cancer cell-derived exosomes stimulate macrophage polarization that creates favorable conditions for LN metastatic processes in TNBC.
Keywords: exosome; lymph node; macrophage; metastasis; triple-negative breast cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no competing interests.
Figures
Similar articles
-
Tumor-derived exosomes in the regulation of macrophage polarization.Inflamm Res. 2020 May;69(5):435-451. doi: 10.1007/s00011-020-01318-0. Epub 2020 Mar 11. Inflamm Res. 2020. PMID: 32162012 Review.
-
Tumor-derived exosomal miR-148b-3p mediates M2 macrophage polarization via TSC2/mTORC1 to promote breast cancer migration and invasion.Thorac Cancer. 2023 Jun;14(16):1477-1491. doi: 10.1111/1759-7714.14891. Epub 2023 May 5. Thorac Cancer. 2023. PMID: 37144254 Free PMC article.
-
Hyperthermia promotes M1 polarization of macrophages via exosome-mediated HSPB8 transfer in triple negative breast cancer.Discov Oncol. 2023 May 26;14(1):81. doi: 10.1007/s12672-023-00697-0. Discov Oncol. 2023. PMID: 37233869 Free PMC article.
-
RAB5A in triple-negative breast cancer: a critical role in macrophage reshaping in an exosomal miR-21-dependent manner.Endocr Relat Cancer. 2024 Apr 12;31(5):e230257. doi: 10.1530/ERC-23-0257. Print 2024 May 1. Endocr Relat Cancer. 2024. PMID: 38470169 Free PMC article.
-
Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models.Adv Drug Deliv Rev. 2013 Mar;65(3):383-90. doi: 10.1016/j.addr.2012.08.007. Epub 2012 Aug 17. Adv Drug Deliv Rev. 2013. PMID: 22921594 Review.
Cited by
-
Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis.Acta Pharm Sin B. 2021 Aug;11(8):2136-2149. doi: 10.1016/j.apsb.2021.04.012. Epub 2021 Apr 20. Acta Pharm Sin B. 2021. PMID: 34522581 Free PMC article. Review.
-
A paclitaxel prodrug nanoparticles with glutathion/reactive oxygen species dual-responsive and CD206 targeting to improve the anti-tumour effect.IET Nanobiotechnol. 2023 Jul;17(5):406-419. doi: 10.1049/nbt2.12119. Epub 2023 Apr 13. IET Nanobiotechnol. 2023. PMID: 37055350 Free PMC article.
-
Exosomal circRHCG promotes breast cancer metastasis via facilitating M2 polarization through TFEB ubiquitination and degradation.NPJ Precis Oncol. 2024 Jan 29;8(1):22. doi: 10.1038/s41698-024-00507-y. NPJ Precis Oncol. 2024. PMID: 38287113 Free PMC article.
-
Tumor-derived exosomes in the regulation of macrophage polarization.Inflamm Res. 2020 May;69(5):435-451. doi: 10.1007/s00011-020-01318-0. Epub 2020 Mar 11. Inflamm Res. 2020. PMID: 32162012 Review.
-
Understanding the tumour micro-environment communication network from an NOS2/COX2 perspective.Br J Pharmacol. 2019 Jan;176(2):155-176. doi: 10.1111/bph.14488. Epub 2018 Nov 6. Br J Pharmacol. 2019. PMID: 30152521 Free PMC article. Review.
References
-
- Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F, ESMO Guidelines Committee Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v8–30. - PubMed
-
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. - PubMed
-
- Kaufmann M, Rody A. Long-term risk of breast cancer recurrence: the need for extended adjuvant therapy. J Cancer Res Clin Oncol. 2005;131:487–494. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

