RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome
- PMID: 29483269
- PMCID: PMC5856561
- DOI: 10.1073/pnas.1800038115
RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome
Abstract
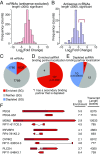
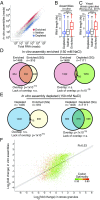
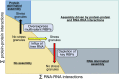
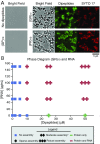
Stress granules are higher order assemblies of nontranslating mRNAs and proteins that form when translation initiation is inhibited. Stress granules are thought to form by protein-protein interactions of RNA-binding proteins. We demonstrate RNA homopolymers or purified cellular RNA forms assemblies in vitro analogous to stress granules. Remarkably, under conditions representative of an intracellular stress response, the mRNAs enriched in assemblies from total yeast RNA largely recapitulate the stress granule transcriptome. We suggest stress granules are formed by a summation of protein-protein and RNA-RNA interactions, with RNA self-assembly likely to contribute to other RNP assemblies wherever there is a high local concentration of RNA. RNA assembly in vitro is also increased by GR and PR dipeptide repeats, which are known to increase stress granule formation in cells. Since GR and PR dipeptides are involved in neurodegenerative diseases, this suggests that perturbations increasing RNA-RNA assembly in cells could lead to disease.
Keywords: RNA self-assembly; RNP granules; dipeptides; stress granules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules.Mol Cell. 2017 Nov 16;68(4):808-820.e5. doi: 10.1016/j.molcel.2017.10.015. Epub 2017 Nov 9. Mol Cell. 2017. PMID: 29129640 Free PMC article.
-
Analyzing P-bodies and stress granules in Saccharomyces cerevisiae.Methods Enzymol. 2010;470:619-40. doi: 10.1016/S0076-6879(10)70025-2. Epub 2010 Mar 1. Methods Enzymol. 2010. PMID: 20946828
-
Stress-specific composition, assembly and kinetics of stress granules in Saccharomyces cerevisiae.J Cell Sci. 2011 Jan 15;124(Pt 2):228-39. doi: 10.1242/jcs.078444. Epub 2010 Dec 15. J Cell Sci. 2011. PMID: 21172806 Free PMC article.
-
Relationship of GW/P-bodies with stress granules.Adv Exp Med Biol. 2013;768:197-211. doi: 10.1007/978-1-4614-5107-5_12. Adv Exp Med Biol. 2013. PMID: 23224972 Free PMC article. Review.
-
RNA Granules: A View from the RNA Perspective.Molecules. 2020 Jul 8;25(14):3130. doi: 10.3390/molecules25143130. Molecules. 2020. PMID: 32650583 Free PMC article. Review.
Cited by
-
The Integral Role of RNA in Stress Granule Formation and Function.Front Cell Dev Biol. 2021 May 20;9:621779. doi: 10.3389/fcell.2021.621779. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095105 Free PMC article. Review.
-
Clustering of RNA Polymerase II C-Terminal Domain Models upon Phosphorylation.J Phys Chem B. 2024 Oct 24;128(42):10385-10396. doi: 10.1021/acs.jpcb.4c04457. Epub 2024 Oct 12. J Phys Chem B. 2024. PMID: 39395159 Free PMC article.
-
The P Granules of C. elegans: A Genetic Model for the Study of RNA-Protein Condensates.J Mol Biol. 2018 Nov 2;430(23):4702-4710. doi: 10.1016/j.jmb.2018.08.007. Epub 2018 Aug 8. J Mol Biol. 2018. PMID: 30096346 Free PMC article. Review.
-
A Heterologous Cell Model for Studying the Role of T-Cell Intracellular Antigen 1 in Welander Distal Myopathy.Mol Cell Biol. 2018 Dec 11;39(1):e00299-18. doi: 10.1128/MCB.00299-18. Print 2019 Jan 1. Mol Cell Biol. 2018. PMID: 30348840 Free PMC article.
-
Characterization of RNA content in individual phase-separated coacervate microdroplets.Nat Commun. 2022 May 12;13(1):2626. doi: 10.1038/s41467-022-30158-1. Nat Commun. 2022. PMID: 35551426 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

