Body temperature measurement in mice during acute illness: implantable temperature transponder versus surface infrared thermometry
- PMID: 29476115
- PMCID: PMC5824949
- DOI: 10.1038/s41598-018-22020-6
Body temperature measurement in mice during acute illness: implantable temperature transponder versus surface infrared thermometry
Abstract
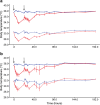
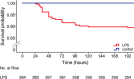
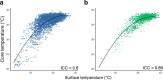
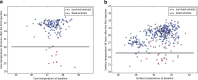
Body temperature is a valuable parameter in determining the wellbeing of laboratory animals. However, using body temperature to refine humane endpoints during acute illness generally lacks comprehensiveness and exposes to inter-observer bias. Here we compared two methods to assess body temperature in mice, namely implanted radio frequency identification (RFID) temperature transponders (method 1) to non-contact infrared thermometry (method 2) in 435 mice for up to 7 days during normothermia and lipopolysaccharide (LPS) endotoxin-induced hypothermia. There was excellent agreement between core and surface temperature as determined by method 1 and 2, respectively, whereas the intra- and inter-subject variation was higher for method 2. Nevertheless, using machine learning algorithms to determine temperature-based endpoints both methods had excellent accuracy in predicting death as an outcome event. Therefore, less expensive and cumbersome non-contact infrared thermometry can serve as a reliable alternative for implantable transponder-based systems for hypothermic responses, although requiring standardization between experimenters.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Comparison of Digital Rectal and Microchip Transponder Thermometry in Ferrets (Mustela putorius furo).J Am Assoc Lab Anim Sci. 2016;55(3):331-5. J Am Assoc Lab Anim Sci. 2016. PMID: 27177569 Free PMC article.
-
Comparison of tympanic, transponder, and noncontact infrared laser thermometry with rectal thermometry in strain 13 guinea pigs (Cavia porcellus).Contemp Top Lab Anim Sci. 2005 Sep;44(5):35-8. Contemp Top Lab Anim Sci. 2005. PMID: 16138780
-
Pre-hospital core temperature measurement in accidental and therapeutic hypothermia.High Alt Med Biol. 2014 Jun;15(2):104-11. doi: 10.1089/ham.2014.1008. Epub 2014 Jun 20. High Alt Med Biol. 2014. PMID: 24950388 Review.
-
Comparison of Microchip Transponder and Noncontact Infrared Thermometry with Rectal Thermometry in Domestic Swine (Sus scrofa domestica).J Am Assoc Lab Anim Sci. 2016;55(5):588-93. J Am Assoc Lab Anim Sci. 2016. PMID: 27657715 Free PMC article.
-
Emergency nursing resource: non-invasive temperature measurement in the emergency department.J Emerg Nurs. 2012 Nov;38(6):523-30. doi: 10.1016/j.jen.2012.05.012. Epub 2012 Oct 4. J Emerg Nurs. 2012. PMID: 23040166 Review. No abstract available.
Cited by
-
Assessing spatial learning and memory in mice: Classic radial maze versus a new animal-friendly automated radial maze allowing free access and not requiring food deprivation.Front Behav Neurosci. 2022 Sep 30;16:1013624. doi: 10.3389/fnbeh.2022.1013624. eCollection 2022. Front Behav Neurosci. 2022. PMID: 36248032 Free PMC article.
-
Simultaneous CMOS-Based Imaging of Calcium Signaling of the Central Amygdala and the Dorsal Raphe Nucleus During Nociception in Freely Moving Mice.Front Neurosci. 2021 May 21;15:667708. doi: 10.3389/fnins.2021.667708. eCollection 2021. Front Neurosci. 2021. PMID: 34135728 Free PMC article.
-
Computational analysis of continuous body temperature provides early discrimination of graft-versus-host disease in mice.Blood Adv. 2019 Dec 10;3(23):3977-3981. doi: 10.1182/bloodadvances.2019000613. Blood Adv. 2019. PMID: 31809535 Free PMC article.
-
The ubiquitin ligase HOIL-1L regulates immune responses by interacting with linear ubiquitin chains.iScience. 2021 Oct 8;24(11):103241. doi: 10.1016/j.isci.2021.103241. eCollection 2021 Nov 19. iScience. 2021. PMID: 34755089 Free PMC article.
-
Thermoregulatory dynamics reveal sex-specific inflammatory responses to experimental autoimmune encephalomyelitis in mice: Implications for multiple sclerosis-induced fatigue in females.Brain Behav Immun Health. 2022 May 31;23:100477. doi: 10.1016/j.bbih.2022.100477. eCollection 2022 Aug. Brain Behav Immun Health. 2022. PMID: 35677535 Free PMC article.
References
-
- Russell, W. M. S. & Burch, R. L. The principles of humane experimental technique. (Methuen, 1959).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

