Relevance of Phosphorylation and Truncation of Tau to the Etiopathogenesis of Alzheimer's Disease
- PMID: 29472853
- PMCID: PMC5810298
- DOI: 10.3389/fnagi.2018.00027
Relevance of Phosphorylation and Truncation of Tau to the Etiopathogenesis of Alzheimer's Disease
Abstract
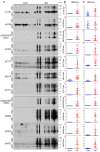
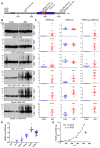
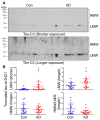
Microtubule (MT) associated protein tau is abnormally hyperphosphorylated and aggregated into paired helical filaments (PHFs), which manifest as neurofibrillary tangles (NFTs) in the brains of individuals with Alzheimer's disease (AD) and related tauopathies. Hyperphosphorylation and truncation of tau have been linked to the progression of the disease. However, the nature of phosphorylation and truncation of tau in AD brain are not very clear. In the present study we investigated the association of phosphorylation and truncation with high-molecular weight oligomers of tau (HMW-tau) in post-mortem AD brain by western blots. We found that tau from AD brain appears as a smear from low molecular weight (LMW) to HMW tau species in western blots developed with pan-tau antibodies. Similar level of LMW-tau was found in AD and control brains, whereas HMW-tau was found in AD brain only. HMW-tau was hyperphosphorylated at multiple sites and not unphosphorylated at Ser46 or Ser198/199/202. HMW-tau was weakly labeled by tau antibodies 43D against a.a. 6-18 and HT7 against a.a. 159-163 of tau, whereas, the C-terminal antibodies, tau46 and tau46.1, strongly labeled HMW-tau. The ratio of HMW-tau/LMW-tau detected by tau antibodies increased as the epitope of the tau antibodies ranges from N-terminal to C-terminal. The level of tau truncated at Asp421 was increased in AD brain, but was poorly associated with the HMW-tau. These findings suggest that tau pathogenesis involves both hyperphosphorylation and dominantly N-terminal truncation of tau in AD.
Keywords: Alzheimer’s disease; hyperphosphorylation; tau; tau pathogenesis; truncation.
Figures


Similar articles
-
Pathological Alterations of Tau in Alzheimer's Disease and 3xTg-AD Mouse Brains.Mol Neurobiol. 2019 Sep;56(9):6168-6183. doi: 10.1007/s12035-019-1507-4. Epub 2019 Feb 8. Mol Neurobiol. 2019. PMID: 30734228
-
The relationship between truncation and phosphorylation at the C-terminus of tau protein in the paired helical filaments of Alzheimer's disease.Front Neurosci. 2015 Feb 11;9:33. doi: 10.3389/fnins.2015.00033. eCollection 2015. Front Neurosci. 2015. PMID: 25717290 Free PMC article.
-
PHF-Core Tau as the Potential Initiating Event for Tau Pathology in Alzheimer's Disease.Front Cell Neurosci. 2020 Sep 10;14:247. doi: 10.3389/fncel.2020.00247. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33132840 Free PMC article.
-
Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
-
Novel drugs affecting tau behavior in the treatment of Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2011 Sep;8(6):678-85. doi: 10.2174/156720511796717122. Curr Alzheimer Res. 2011. PMID: 21605038 Review.
Cited by
-
The Role of Copper in Tau-Related Pathology in Alzheimer's Disease.Front Mol Neurosci. 2020 Sep 10;13:572308. doi: 10.3389/fnmol.2020.572308. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33071757 Free PMC article. Review.
-
A walk through tau therapeutic strategies.Acta Neuropathol Commun. 2019 Feb 15;7(1):22. doi: 10.1186/s40478-019-0664-z. Acta Neuropathol Commun. 2019. PMID: 30767766 Free PMC article. Review.
-
Distinct dual roles of p-Tyr42 RhoA GTPase in tau phosphorylation and ATP citrate lyase activation upon different Aβ concentrations.Redox Biol. 2020 May;32:101446. doi: 10.1016/j.redox.2020.101446. Epub 2020 Jan 31. Redox Biol. 2020. PMID: 32046944 Free PMC article.
-
Epigallocatechin-3-gallate modulates Tau Post-translational modifications and cytoskeletal network.Oncotarget. 2021 May 25;12(11):1083-1099. doi: 10.18632/oncotarget.27963. eCollection 2021 May 25. Oncotarget. 2021. PMID: 34084282 Free PMC article.
-
Tau Filament Self-Assembly and Structure: Tau as a Therapeutic Target.Front Neurol. 2020 Nov 12;11:590754. doi: 10.3389/fneur.2020.590754. eCollection 2020. Front Neurol. 2020. PMID: 33281730 Free PMC article. Review.
References
-
- Abraha A., Ghoshal N., Gamblin T. C., Cryns V., Berry R. W., Kuret J., et al. . (2000). C-terminal inhibition of tau assembly in vitro and in Alzheimer’s disease. J. Cell Sci. 113, 3737–3745. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

