Structure of full-length human TRPM4
- PMID: 29463718
- PMCID: PMC5877947
- DOI: 10.1073/pnas.1722038115
Structure of full-length human TRPM4
Abstract
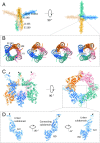
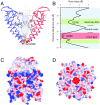
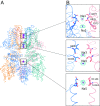
Transient receptor potential melastatin subfamily member 4 (TRPM4) is a widely distributed, calcium-activated, monovalent-selective cation channel. Mutations in human TRPM4 (hTRPM4) result in progressive familial heart block. Here, we report the electron cryomicroscopy structure of hTRPM4 in a closed, Na+-bound, apo state at pH 7.5 to an overall resolution of 3.7 Å. Five partially hydrated sodium ions are proposed to occupy the center of the conduction pore and the entrance to the coiled-coil domain. We identify an upper gate in the selectivity filter and a lower gate at the entrance to the cytoplasmic coiled-coil domain. Intramolecular interactions exist between the TRP domain and the S4-S5 linker, N-terminal domain, and N and C termini. Finally, we identify aromatic interactions via π-π bonds and cation-π bonds, glycosylation at an N-linked extracellular site, a pore-loop disulfide bond, and 24 lipid binding sites. We compare and contrast this structure with other TRP channels and discuss potential mechanisms of regulation and gating of human full-length TRPM4.
Keywords: cardiac arrhythmia; cryomicroscopy; ion channel; transient receptor potential channel.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


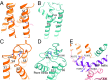
Similar articles
-
Structure of the human TRPM4 ion channel in a lipid nanodisc.Science. 2018 Jan 12;359(6372):228-232. doi: 10.1126/science.aar4510. Epub 2017 Dec 7. Science. 2018. PMID: 29217581 Free PMC article.
-
Structures of the calcium-activated, non-selective cation channel TRPM4.Nature. 2017 Dec 14;552(7684):205-209. doi: 10.1038/nature24997. Epub 2017 Dec 6. Nature. 2017. PMID: 29211714 Free PMC article.
-
Transient receptor potential melastatin 4 cation channel in pediatric heart block.Eur Rev Med Pharmacol Sci. 2017 Oct;21(4 Suppl):79-84. Eur Rev Med Pharmacol Sci. 2017. PMID: 29165759
-
TRPM4.Handb Exp Pharmacol. 2014;222:461-87. doi: 10.1007/978-3-642-54215-2_18. Handb Exp Pharmacol. 2014. PMID: 24756717 Review.
-
Regulation of TRP channels: a voltage-lipid connection.Biochem Soc Trans. 2007 Feb;35(Pt 1):105-8. doi: 10.1042/BST0350105. Biochem Soc Trans. 2007. PMID: 17233613 Review.
Cited by
-
Mapping of CaM, S100A1 and PIP2-Binding Epitopes in the Intracellular N- and C-Termini of TRPM4.Int J Mol Sci. 2020 Jun 17;21(12):4323. doi: 10.3390/ijms21124323. Int J Mol Sci. 2020. PMID: 32560560 Free PMC article.
-
Structural mechanism of TRPM7 channel regulation by intracellular magnesium.Cell Mol Life Sci. 2022 Apr 7;79(5):225. doi: 10.1007/s00018-022-04192-7. Cell Mol Life Sci. 2022. PMID: 35389104 Free PMC article.
-
Conformational ensemble of the human TRPV3 ion channel.Nat Commun. 2018 Nov 14;9(1):4773. doi: 10.1038/s41467-018-07117-w. Nat Commun. 2018. PMID: 30429472 Free PMC article.
-
TRP ion channels: Proteins with conformational flexibility.Channels (Austin). 2019 Dec;13(1):207-226. doi: 10.1080/19336950.2019.1626793. Channels (Austin). 2019. PMID: 31184289 Free PMC article. Review.
-
Structural dynamics at cytosolic interprotomer interfaces control gating of a mammalian TRPM5 channel.Proc Natl Acad Sci U S A. 2024 Jul 2;121(27):e2403333121. doi: 10.1073/pnas.2403333121. Epub 2024 Jun 26. Proc Natl Acad Sci U S A. 2024. PMID: 38923985 Free PMC article.
References
-
- Clapham DE, Runnels LW, Strübing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. - PubMed
-
- Launay P, et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. - PubMed
-
- Nilius B, et al. The selectivity filter of the cation channel TRPM4. J Biol Chem. 2005;280:22899–22906. - PubMed
-
- Nilius B, et al. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

