Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor
- PMID: 29454749
- PMCID: PMC5906738
- DOI: 10.1016/j.ajpath.2018.01.011
Microbiota-Derived Indole Metabolites Promote Human and Murine Intestinal Homeostasis through Regulation of Interleukin-10 Receptor
Abstract
Interactions between the gut microbiota and the host are important for health, where dysbiosis has emerged as a likely component of mucosal disease. The specific constituents of the microbiota that contribute to mucosal disease are not well defined. The authors sought to define microbial components that regulate homeostasis within the intestinal mucosa. Using an unbiased, metabolomic profiling approach, a selective depletion of indole and indole-derived metabolites was identified in murine and human colitis. Indole-3-propionic acid (IPA) was selectively diminished in circulating serum from human subjects with active colitis, and IPA served as a biomarker of disease remission. Administration of indole metabolites showed prominent induction of IL-10R1 on cultured intestinal epithelia that was explained by activation of the aryl hydrocarbon receptor. Colonization of germ-free mice with wild-type Escherichia coli, but not E. coli mutants unable to generate indole, induced colonic epithelial IL-10R1. Moreover, oral administration of IPA significantly ameliorated disease in a chemically induced murine colitis model. This work defines a novel role of indole metabolites in anti-inflammatory pathways mediated by epithelial IL-10 signaling and identifies possible avenues for utilizing indoles as novel therapeutics in mucosal disease.
Copyright © 2018 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
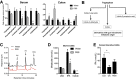
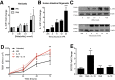
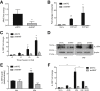
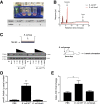

Similar articles
-
Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia.Mucosal Immunol. 2017 Sep;10(5):1133-1144. doi: 10.1038/mi.2016.133. Epub 2017 Jan 18. Mucosal Immunol. 2017. PMID: 28098246 Free PMC article.
-
The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy.Gut Microbes. 2016 May 3;7(3):246-61. doi: 10.1080/19490976.2016.1156827. Epub 2016 Mar 23. Gut Microbes. 2016. PMID: 27007819 Free PMC article.
-
Mucosa repair mechanisms of Tong-Xie-Yao-Fang mediated by CRH-R2 in murine, dextran sulfate sodium-induced colitis.World J Gastroenterol. 2018 Apr 28;24(16):1766-1778. doi: 10.3748/wjg.v24.i16.1766. World J Gastroenterol. 2018. PMID: 29713130 Free PMC article.
-
Indole compounds may be promising medicines for ulcerative colitis.J Gastroenterol. 2016 Sep;51(9):853-61. doi: 10.1007/s00535-016-1220-2. Epub 2016 May 9. J Gastroenterol. 2016. PMID: 27160749 Review.
-
Xenobiotic receptors and the regulation of intestinal homeostasis: harnessing the chemical output of the intestinal microbiota.Am J Physiol Gastrointest Liver Physiol. 2022 Feb 1;322(2):G268-G281. doi: 10.1152/ajpgi.00160.2021. Epub 2021 Dec 23. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 34941453 Review.
Cited by
-
Type 1 diabetes: immune pathology and novel therapeutic approaches.Diabetol Int. 2024 Sep 11;15(4):761-776. doi: 10.1007/s13340-024-00748-z. eCollection 2024 Oct. Diabetol Int. 2024. PMID: 39469552 Review.
-
The Gut Microbiota Modulates Neuroinflammation in Alzheimer's Disease: Elucidating Crucial Factors and Mechanistic Underpinnings.CNS Neurosci Ther. 2024 Oct;30(10):e70091. doi: 10.1111/cns.70091. CNS Neurosci Ther. 2024. PMID: 39460538 Free PMC article. Review.
-
Indole-3-Acetic Acid Esterified with Waxy, Normal, and High-Amylose Maize Starches: Comparative Study on Colon-Targeted Delivery and Intestinal Health Impact.Nutrients. 2024 Oct 11;16(20):3446. doi: 10.3390/nu16203446. Nutrients. 2024. PMID: 39458442 Free PMC article.
-
New insights into the intestinal barrier through "gut-organ" axes and a glimpse of the microgravity's effects on intestinal barrier.Front Physiol. 2024 Oct 10;15:1465649. doi: 10.3389/fphys.2024.1465649. eCollection 2024. Front Physiol. 2024. PMID: 39450142 Free PMC article. Review.
-
Clostridium sporogenes-derived metabolites protect mice against colonic inflammation.Gut Microbes. 2024 Jan-Dec;16(1):2412669. doi: 10.1080/19490976.2024.2412669. Epub 2024 Oct 14. Gut Microbes. 2024. PMID: 39397690 Free PMC article.
References
-
- Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

