Targeting KCa1.1 Channels with a Scorpion Venom Peptide for the Therapy of Rat Models of Rheumatoid Arthritis
- PMID: 29453198
- PMCID: PMC5878672
- DOI: 10.1124/jpet.117.245118
Targeting KCa1.1 Channels with a Scorpion Venom Peptide for the Therapy of Rat Models of Rheumatoid Arthritis
Abstract
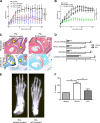
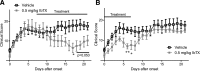
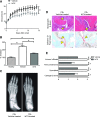
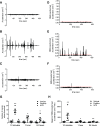
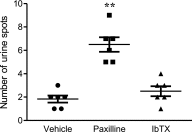
Fibroblast-like synoviocytes (FLSs) are a key cell type involved in rheumatoid arthritis (RA) progression. We previously identified the KCa1.1 potassium channel (Maxi-K, BK, Slo 1, KCNMA1) as a regulator of FLSs and found that KCa1.1 inhibition reduces disease severity in RA animal models. However, systemic KCa1.1 block causes multiple side effects. In this study, we aimed to determine whether the KCa1.1 β1-3-specific venom peptide blocker iberiotoxin (IbTX) reduces disease severity in animal models of RA without inducing major side effects. We used immunohistochemistry to identify IbTX-sensitive KCa1.1 subunits in joints of rats with a model of RA. Patch-clamp and functional assays were used to determine whether IbTX can regulate FLSs through targeting KCa1.1. We then tested the efficacy of IbTX in ameliorating disease in two rat models of RA. Finally, we determined whether IbTX causes side effects including incontinence or tremors in rats, compared with those treated with the small-molecule KCa1.1 blocker paxilline. IbTX-sensitive subunits of KCa1.1 were expressed by FLSs in joints of rats with experimental arthritis. IbTX inhibited KCa1.1 channels expressed by FLSs from patients with RA and by FLSs from rat models of RA and reduced FLS invasiveness. IbTX significantly reduced disease severity in two rat models of RA. Unlike paxilline, IbTX did not induce tremors or incontinence in rats. Overall, IbTX inhibited KCa1.1 channels on FLSs and treated rat models of RA without inducing side effects associated with nonspecific KCa1.1 blockade and could become the basis for the development of a new treatment of RA.
Copyright © 2018 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


Similar articles
-
Exploring the therapeutic opportunities of potassium channels for the treatment of rheumatoid arthritis.Front Pharmacol. 2024 May 9;15:1286069. doi: 10.3389/fphar.2024.1286069. eCollection 2024. Front Pharmacol. 2024. PMID: 38783950 Free PMC article. Review.
-
Different expression of β subunits of the KCa1.1 channel by invasive and non-invasive human fibroblast-like synoviocytes.Arthritis Res Ther. 2016 May 10;18(1):103. doi: 10.1186/s13075-016-1003-4. Arthritis Res Ther. 2016. PMID: 27165430 Free PMC article.
-
KCa1.1 inhibition attenuates fibroblast-like synoviocyte invasiveness and ameliorates disease in rat models of rheumatoid arthritis.Arthritis Rheumatol. 2015 Jan;67(1):96-106. doi: 10.1002/art.38883. Arthritis Rheumatol. 2015. PMID: 25252152 Free PMC article.
-
KCa1.1 channels regulate β1-integrin function and cell adhesion in rheumatoid arthritis fibroblast-like synoviocytes.FASEB J. 2017 Aug;31(8):3309-3320. doi: 10.1096/fj.201601097R. Epub 2017 Apr 20. FASEB J. 2017. PMID: 28428266 Free PMC article.
-
Role of glucose metabolism in aggressive phenotype of fibroblast-like synoviocytes: Latest evidence and therapeutic approaches in rheumatoid arthritis.Int Immunopharmacol. 2020 Dec;89(Pt A):107064. doi: 10.1016/j.intimp.2020.107064. Epub 2020 Oct 8. Int Immunopharmacol. 2020. PMID: 33039953 Review.
Cited by
-
The Bi-Functional Paxilline Enriched in Skin Secretion of Tree Frogs (Hyla japonica) Targets the KCNK18 and BKCa Channels.Toxins (Basel). 2023 Jan 12;15(1):70. doi: 10.3390/toxins15010070. Toxins (Basel). 2023. PMID: 36668889 Free PMC article.
-
Exploring the therapeutic opportunities of potassium channels for the treatment of rheumatoid arthritis.Front Pharmacol. 2024 May 9;15:1286069. doi: 10.3389/fphar.2024.1286069. eCollection 2024. Front Pharmacol. 2024. PMID: 38783950 Free PMC article. Review.
-
Immunomodulatory effect of Tityus sp. in mononuclear cells extracted from the blood of rheumatoid arthritis patients.J Venom Anim Toxins Incl Trop Dis. 2024 Sep 16;30:e20230064. doi: 10.1590/1678-9199-JVATITD-2023-0064. eCollection 2024. J Venom Anim Toxins Incl Trop Dis. 2024. PMID: 39445068 Free PMC article.
-
Mapping the functional expression of auxiliary subunits of KCa1.1 in glioblastoma.Sci Rep. 2022 Dec 20;12(1):22023. doi: 10.1038/s41598-022-26196-w. Sci Rep. 2022. PMID: 36539587 Free PMC article.
-
Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System.Front Mol Neurosci. 2018 Jul 30;11:258. doi: 10.3389/fnmol.2018.00258. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30104956 Free PMC article. Review.
References
-
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham Co, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581. - PubMed
-
- Arkett SA, Dixon J, Yang JN, Sakai DD, Minkin C, Sims SM. (1994) Mammalian osteoclasts express a transient potassium channel with properties of Kv1.3. Receptors Channels 2:281–293. - PubMed
-
- Beeton C, Chandy KG. (2005) Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist 11:550–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
