High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice
- PMID: 29440390
- PMCID: PMC5900752
- DOI: 10.1074/jbc.RA117.000808
High dietary fat and sucrose results in an extensive and time-dependent deterioration in health of multiple physiological systems in mice
Abstract
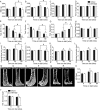
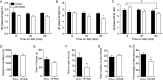
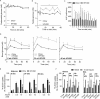
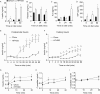
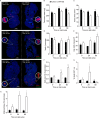
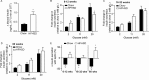
Obesity is associated with metabolic dysfunction, including insulin resistance and hyperinsulinemia, and with disorders such as cardiovascular disease, osteoporosis, and neurodegeneration. Typically, these pathologies are examined in discrete model systems and with limited temporal resolution, and whether these disorders co-occur is therefore unclear. To address this question, here we examined multiple physiological systems in male C57BL/6J mice following prolonged exposure to a high-fat/high-sucrose diet (HFHSD). HFHSD-fed mice rapidly exhibited metabolic alterations, including obesity, hyperleptinemia, physical inactivity, glucose intolerance, peripheral insulin resistance, fasting hyperglycemia, ectopic lipid deposition, and bone deterioration. Prolonged exposure to HFHSD resulted in morbid obesity, ectopic triglyceride deposition in liver and muscle, extensive bone loss, sarcopenia, hyperinsulinemia, and impaired short-term memory. Although many of these defects are typically associated with aging, HFHSD did not alter telomere length in white blood cells, indicating that this diet did not generally promote all aspects of aging. Strikingly, glucose homeostasis was highly dynamic. Glucose intolerance was evident in HFHSD-fed mice after 1 week and was maintained for 24 weeks. Beyond 24 weeks, however, glucose tolerance improved in HFHSD-fed mice, and by 60 weeks, it was indistinguishable from that of chow-fed mice. This improvement coincided with adaptive β-cell hyperplasia and hyperinsulinemia, without changes in insulin sensitivity in muscle or adipose tissue. Assessment of insulin secretion in isolated islets revealed that leptin, which inhibited insulin secretion in the chow-fed mice, potentiated glucose-stimulated insulin secretion in the HFHSD-fed mice after 60 weeks. Overall, the excessive calorie intake was accompanied by deteriorating function of numerous physiological systems.
Keywords: Western diet; beta cell (B-cell); bone; diabetes; glucose metabolism; insulin secretion; leptin; neurodegeneration; obesity.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Resolution of glucose intolerance in long-term high-fat, high-sucrose-fed mice.J Endocrinol. 2017 Jun;233(3):269-279. doi: 10.1530/JOE-17-0004. Epub 2017 Mar 30. J Endocrinol. 2017. PMID: 28360081
-
Timing of Medium-Chain Triglyceride Consumption Modulates Effects in Mice with Obesity Induced by a High-Fat High-Sucrose Diet.Nutrients. 2022 Dec 1;14(23):5096. doi: 10.3390/nu14235096. Nutrients. 2022. PMID: 36501131 Free PMC article.
-
Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice.Metabolism. 1995 May;44(5):645-51. doi: 10.1016/0026-0495(95)90123-x. Metabolism. 1995. PMID: 7752914
-
Distinct Influence of Hypercaloric Diets Predominant with Fat or Fat and Sucrose on Adipose Tissue and Liver Inflammation in Mice.Molecules. 2020 Sep 23;25(19):4369. doi: 10.3390/molecules25194369. Molecules. 2020. PMID: 32977558 Free PMC article.
-
Changes in adiponectin, its receptors and AMPK activity in tissues of diet-induced diabetic mice.Diabetes Metab. 2008 Feb;34(1):52-61. doi: 10.1016/j.diabet.2007.09.006. Epub 2008 Jan 25. Diabetes Metab. 2008. PMID: 18222103
Cited by
-
Hyperglycaemia is associated with impaired muscle signalling and aerobic adaptation to exercise.Nat Metab. 2020 Sep;2(9):902-917. doi: 10.1038/s42255-020-0240-7. Epub 2020 Jul 20. Nat Metab. 2020. PMID: 32694831 Free PMC article.
-
Insulin sensitivity is preserved in mice made obese by feeding a high starch diet.Elife. 2022 Nov 17;11:e79250. doi: 10.7554/eLife.79250. Elife. 2022. PMID: 36394259 Free PMC article.
-
Telomere length in leucocytes and solid tissues of young and aged rats.Aging (Albany NY). 2022 Feb 27;14(4):1713-1728. doi: 10.18632/aging.203922. Epub 2022 Feb 27. Aging (Albany NY). 2022. PMID: 35220278 Free PMC article.
-
Insulin Tolerance Test under Anaesthesia to Measure Tissue-specific Insulin-stimulated Glucose Disposal.Bio Protoc. 2019 Jan 20;9(2):e3146. doi: 10.21769/BioProtoc.3146. eCollection 2019 Jan 20. Bio Protoc. 2019. PMID: 33654891 Free PMC article.
-
Sargassum fusiforme Polysaccharides Prevent High-Fat Diet-Induced Early Fasting Hypoglycemia and Regulate the Gut Microbiota Composition.Mar Drugs. 2020 Aug 27;18(9):444. doi: 10.3390/md18090444. Mar Drugs. 2020. PMID: 32867177 Free PMC article.
References
-
- Solon-Biet S. M., McMahon A. C., Ballard J. W., Ruohonen K., Wu L. E., Cogger V. C., Warren A., Huang X., Pichaud N., Melvin R. G., Gokarn R., Khalil M., Turner N., Cooney G. J., Sinclair D. A., et al. (2014) The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 19, 418–430 10.1016/j.cmet.2014.02.009 - DOI - PMC - PubMed
-
- Barclay J. L., Shostak A., Leliavski A., Tsang A. H., Jöhren O., Müller-Fielitz H., Landgraf D., Naujokat N., van der Horst G. T., and Oster H. (2013) High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am. J. Physiol. Endocrinol. Metab. 304, E1053–E1063 10.1152/ajpendo.00512.2012 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

