Fentanyl Induces Rapid Onset Hyperalgesic Priming: Type I at Peripheral and Type II at Central Nociceptor Terminals
- PMID: 29431655
- PMCID: PMC5830512
- DOI: 10.1523/JNEUROSCI.3476-17.2018
Fentanyl Induces Rapid Onset Hyperalgesic Priming: Type I at Peripheral and Type II at Central Nociceptor Terminals
Abstract
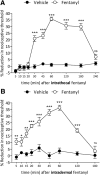
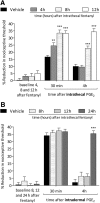
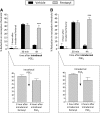
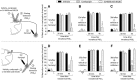
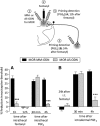
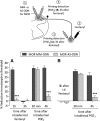
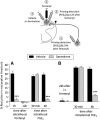
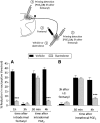
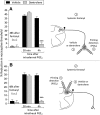
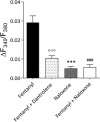
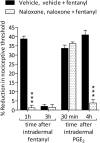
Systemic fentanyl induces hyperalgesic priming, long-lasting neuroplasticity in nociceptor function characterized by prolongation of inflammatory mediator hyperalgesia. To evaluate priming at both nociceptor terminals, we studied, in male Sprague Dawley rats, the effect of local administration of agents that reverse type I (protein translation) or type II [combination of Src and mitogen-activated protein kinase (MAPK)] priming. At the central terminal, priming induced by systemic, intradermal, or intrathecal fentanyl was reversed by the combination of Src and MAPK inhibitors, but at the peripheral terminal, it was reversed by the protein translation inhibitor. Mu-opioid receptor (MOR) antisense prevented fentanyl hyperalgesia and priming. To determine whether type I and II priming occur in the same population of neurons, we used isolectin B4-saporin or [Sar9, Met(O2)11]-substance P-saporin to deplete nonpeptidergic or peptidergic nociceptors, respectively. Following intrathecal fentanyl, central terminal priming was prevented by both saporins, whereas that in peripheral terminal was not attenuated even by their combination. However, after intradermal fentanyl, priming in the peripheral terminal requires both peptidergic and nonpeptidergic nociceptors, whereas that in the central terminal is dependent only on peptidergic nociceptors. Pretreatment with dantrolene at either terminal prevented fentanyl-induced priming in both terminals, suggesting communication between central and peripheral terminals mediated by intracellular Ca2+ signaling. In vitro application of fentanyl increased cytoplasmic Ca2+ concentration in dorsal root ganglion neurons, which was prevented by pretreatment with dantrolene and naloxone. Therefore, acting at MOR in the nociceptor, fentanyl induces hyperalgesia and priming rapidly at both the central (type II) and peripheral (type I) terminal and this is mediated by Ca2+ signaling.SIGNIFICANCE STATEMENT Fentanyl, acting at the μ-opioid receptor (MOR), induces hyperalgesia and hyperalgesic priming at both the central and peripheral terminal of nociceptors and this is mediated by endoplasmic reticulum Ca2+ signaling. Priming in the central terminal is type II, whereas that in the peripheral terminal is type I. Our findings may provide useful information for the design of drugs with improved therapeutic profiles, selectively disrupting individual MOR signaling pathways, to maintain an adequate long-lasting control of pain.
Keywords: calcium; endoplasmic reticulum; fentanyl; hyperalgesia; hyperalgesic priming; μ-opioid receptor (MOR).
Copyright © 2018 the authors 0270-6474/18/382226-20$15.00/0.
Figures



Similar articles
-
Opioid-Induced Hyperalgesic Priming in Single Nociceptors.J Neurosci. 2021 Jan 6;41(1):31-46. doi: 10.1523/JNEUROSCI.2160-20.2020. Epub 2020 Nov 17. J Neurosci. 2021. PMID: 33203743 Free PMC article.
-
In Vitro Nociceptor Neuroplasticity Associated with In Vivo Opioid-Induced Hyperalgesia.J Neurosci. 2019 Sep 4;39(36):7061-7073. doi: 10.1523/JNEUROSCI.1191-19.2019. Epub 2019 Jul 12. J Neurosci. 2019. PMID: 31300521 Free PMC article.
-
Role of Nociceptor Toll-like Receptor 4 (TLR4) in Opioid-Induced Hyperalgesia and Hyperalgesic Priming.J Neurosci. 2019 Aug 14;39(33):6414-6424. doi: 10.1523/JNEUROSCI.0966-19.2019. Epub 2019 Jun 17. J Neurosci. 2019. PMID: 31209174 Free PMC article.
-
The pharmacology of nociceptor priming.Handb Exp Pharmacol. 2015;227:15-37. doi: 10.1007/978-3-662-46450-2_2. Handb Exp Pharmacol. 2015. PMID: 25846612 Free PMC article. Review.
-
Roles of Proton-Sensing Receptors in the Transition from Acute to Chronic Pain.J Dent Res. 2016 Feb;95(2):135-42. doi: 10.1177/0022034515618382. Epub 2015 Nov 23. J Dent Res. 2016. PMID: 26597969 Review.
Cited by
-
Preclinical study in a postoperative pain model to investigate the action of ketamine, lidocaine, and ascorbic acid in reversing fentanyl-induced, non-glutamate-dependent hyperalgesia.Pain Rep. 2023 Feb 13;8(2):e1062. doi: 10.1097/PR9.0000000000001062. eCollection 2023 Mar-Apr. Pain Rep. 2023. PMID: 37731750 Free PMC article.
-
The prolactin receptor long isoform regulates nociceptor sensitization and opioid-induced hyperalgesia selectively in females.Sci Transl Med. 2020 Feb 5;12(529):eaay7550. doi: 10.1126/scitranslmed.aay7550. Sci Transl Med. 2020. PMID: 32024801 Free PMC article.
-
Pharmacological Chaperones Attenuate the Development of Opioid Tolerance.Int J Mol Sci. 2020 Oct 13;21(20):7536. doi: 10.3390/ijms21207536. Int J Mol Sci. 2020. PMID: 33066035 Free PMC article.
-
Systemic Morphine Produces Dose-dependent Nociceptor-mediated Biphasic Changes in Nociceptive Threshold and Neuroplasticity.Neuroscience. 2019 Feb 1;398:64-75. doi: 10.1016/j.neuroscience.2018.11.051. Epub 2018 Dec 7. Neuroscience. 2019. PMID: 30529265 Free PMC article.
-
Endogenous and Exogenous Opioids in Pain.Annu Rev Neurosci. 2018 Jul 8;41:453-473. doi: 10.1146/annurev-neuro-080317-061522. Epub 2018 May 31. Annu Rev Neurosci. 2018. PMID: 29852083 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
