OPA1 gene therapy prevents retinal ganglion cell loss in a Dominant Optic Atrophy mouse model
- PMID: 29410463
- PMCID: PMC5802757
- DOI: 10.1038/s41598-018-20838-8
OPA1 gene therapy prevents retinal ganglion cell loss in a Dominant Optic Atrophy mouse model
Abstract
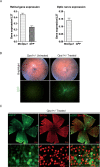
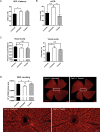
Dominant optic atrophy (DOA) is a rare progressive and irreversible blinding disease which is one of the most frequent forms of hereditary optic neuropathy. DOA is mainly caused by dominant mutation in the OPA1 gene encoding a large mitochondrial GTPase with crucial roles in membrane dynamics and cell survival. Hereditary optic neuropathies are commonly characterized by the degeneration of retinal ganglion cells, leading to the optic nerve atrophy and the progressive loss of visual acuity. Up to now, despite increasing advances in the understanding of the pathological mechanisms, DOA remains intractable. Here, we tested the efficiency of gene therapy on a genetically-modified mouse model reproducing DOA vision loss. We performed intravitreal injections of an Adeno-Associated Virus carrying the human OPA1 cDNA under the control of the cytomegalovirus promotor. Our results provide the first evidence that gene therapy is efficient on a mouse model of DOA as the wild-type OPA1 expression is able to alleviate the OPA1-induced retinal ganglion cell degeneration, the hallmark of the disease. These results displayed encouraging effects of gene therapy for Dominant Optic Atrophy, fostering future investigations aiming at clinical trials in patients.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Dominant optic atrophy: Culprit mitochondria in the optic nerve.Prog Retin Eye Res. 2021 Jul;83:100935. doi: 10.1016/j.preteyeres.2020.100935. Epub 2020 Dec 17. Prog Retin Eye Res. 2021. PMID: 33340656 Review.
-
Increased steroidogenesis promotes early-onset and severe vision loss in females with OPA1 dominant optic atrophy.Hum Mol Genet. 2016 Jun 15;25(12):2539-2551. doi: 10.1093/hmg/ddw117. Epub 2016 Jun 3. Hum Mol Genet. 2016. PMID: 27260406
-
OPA1: How much do we know to approach therapy?Pharmacol Res. 2018 May;131:199-210. doi: 10.1016/j.phrs.2018.02.018. Epub 2018 Feb 15. Pharmacol Res. 2018. PMID: 29454676 Review.
-
Dominant optic atrophy: updates on the pathophysiology and clinical manifestations of the optic atrophy 1 mutation.Curr Opin Ophthalmol. 2016 Nov;27(6):475-480. doi: 10.1097/ICU.0000000000000314. Curr Opin Ophthalmol. 2016. PMID: 27585216 Review.
-
A recurrent deletion mutation in OPA1 causes autosomal dominant optic atrophy in a Chinese family.Sci Rep. 2014 Nov 6;4:6936. doi: 10.1038/srep06936. Sci Rep. 2014. PMID: 25374051 Free PMC article.
Cited by
-
Mitochondrial Dynamics: Molecular Mechanisms, Related Primary Mitochondrial Disorders and Therapeutic Approaches.Genes (Basel). 2021 Feb 10;12(2):247. doi: 10.3390/genes12020247. Genes (Basel). 2021. PMID: 33578638 Free PMC article. Review.
-
Optimized OPA1 Isoforms 1 and 7 Provide Therapeutic Benefit in Models of Mitochondrial Dysfunction.Front Neurosci. 2020 Nov 26;14:571479. doi: 10.3389/fnins.2020.571479. eCollection 2020. Front Neurosci. 2020. PMID: 33324145 Free PMC article.
-
The role of mitochondrial dynamics in disease.MedComm (2020). 2023 Dec 28;4(6):e462. doi: 10.1002/mco2.462. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38156294 Free PMC article. Review.
-
Atherosclerosis as Mitochondriopathy: Repositioning the Disease to Help Finding New Therapies.Front Cardiovasc Med. 2021 May 4;8:660473. doi: 10.3389/fcvm.2021.660473. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34017868 Free PMC article. Review.
-
Autosomal dominant optic atrophy caused by six novel pathogenic OPA1 variants and genotype-phenotype correlation analysis.BMC Ophthalmol. 2022 Jul 26;22(1):322. doi: 10.1186/s12886-022-02546-0. BMC Ophthalmol. 2022. PMID: 35883160 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

