Pacing across the membrane: the novel PACE family of efflux pumps is widespread in Gram-negative pathogens
- PMID: 29409983
- PMCID: PMC6195760
- DOI: 10.1016/j.resmic.2018.01.001
Pacing across the membrane: the novel PACE family of efflux pumps is widespread in Gram-negative pathogens
Abstract
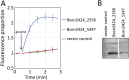
The proteobacterial antimicrobial compound efflux (PACE) family of transport proteins was only recently described. PACE family transport proteins can confer resistance to a range of biocides used as disinfectants and antiseptics, and are encoded by many important Gram-negative human pathogens. However, we are only just beginning to appreciate the range of functions and the mechanism(s) of transport operating in these proteins. Genes encoding PACE family proteins are typically conserved in the core genomes of bacterial species rather than on recently acquired mobile genetic elements, suggesting that they confer important core functions in addition to biocide resistance. Three-dimensional structural information is not yet available for PACE family proteins. However, PACE proteins have several very highly conserved amino acid sequence motifs that are likely to be important for substrate transport. PACE proteins also display strong amino acid sequence conservation between their N and C-terminal halves, suggesting that they evolved by duplication of an ancestral protein comprised of two transmembrane helices. In light of their drug resistance functions in Gram-negative pathogens, PACE proteins should be the subject of detailed future investigation.
Keywords: Antimicrobial resistance; Bacterial transmembrane pair domain; Efflux; Gram-negative pathogen; Membrane transport; PACE.
Copyright © 2018 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Figures
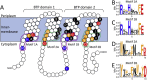
Similar articles
-
Reconstitution of the activity of RND efflux pumps: a "bottom-up" approach.Res Microbiol. 2018 Sep-Oct;169(7-8):442-449. doi: 10.1016/j.resmic.2017.11.004. Epub 2017 Dec 5. Res Microbiol. 2018. PMID: 29217371 Review.
-
Homologs of the Acinetobacter baumannii AceI transporter represent a new family of bacterial multidrug efflux systems.mBio. 2015 Feb 10;6(1):e01982-14. doi: 10.1128/mBio.01982-14. mBio. 2015. PMID: 25670776 Free PMC article.
-
Short-chain diamines are the physiological substrates of PACE family efflux pumps.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):18015-18020. doi: 10.1073/pnas.1901591116. Epub 2019 Aug 15. Proc Natl Acad Sci U S A. 2019. PMID: 31416917 Free PMC article.
-
Fluorescence enlightens RND pump activity and the intrabacterial concentration of antibiotics.Res Microbiol. 2018 Sep-Oct;169(7-8):432-441. doi: 10.1016/j.resmic.2017.11.005. Epub 2017 Dec 5. Res Microbiol. 2018. PMID: 29208490 Review.
-
Molecular mechanisms of AcrB-mediated multidrug export.Res Microbiol. 2018 Sep-Oct;169(7-8):372-383. doi: 10.1016/j.resmic.2018.05.005. Epub 2018 May 25. Res Microbiol. 2018. PMID: 29807096 Review.
Cited by
-
Molecular and phenotypic characterization of efflux pump and biofilm in multi-drug resistant non-typhoidal Salmonella Serovars isolated from food animals and handlers in Lagos Nigeria.One Health Outlook. 2021 Feb 9;3:2. doi: 10.1186/s42522-021-00035-w. eCollection 2021. One Health Outlook. 2021. PMID: 33829140 Free PMC article.
-
Structural mass spectrometry approaches to understand multidrug efflux systems.Essays Biochem. 2023 Mar 29;67(2):255-267. doi: 10.1042/EBC20220190. Essays Biochem. 2023. PMID: 36504255 Free PMC article. Review.
-
The Varied Role of Efflux Pumps of the MFS Family in the Interplay of Bacteria with Animal and Plant Cells.Microorganisms. 2019 Aug 22;7(9):285. doi: 10.3390/microorganisms7090285. Microorganisms. 2019. PMID: 31443538 Free PMC article. Review.
-
Role of Berberine as a Potential Efflux Pump Inhibitor against MdfA from Escherichia coli: In Vitro and In Silico Studies.Microbiol Spectr. 2023 Feb 14;11(2):e0332422. doi: 10.1128/spectrum.03324-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36786641 Free PMC article.
-
Resistance Toward Chlorhexidine in Oral Bacteria - Is There Cause for Concern?Front Microbiol. 2019 Mar 22;10:587. doi: 10.3389/fmicb.2019.00587. eCollection 2019. Front Microbiol. 2019. PMID: 30967854 Free PMC article. Review.
References
-
- Du D., van Veen H.W., Murakami S., Pos K.M., Luisi B.F. Structure, mechanism and cooperation of bacterial multidrug transporters. Curr Opin Struct Biol. 2015;33:76–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

