Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity
- PMID: 29400713
- PMCID: PMC5839993
- DOI: 10.1038/nm.4481
Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity
Abstract
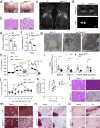
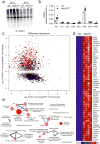
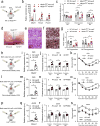
Adipocytes possess remarkable adaptive capacity to respond to nutrient excess, fasting or cold exposure, and they are thus an important cell type for the maintenance of proper metabolic health. Although the endoplasmic reticulum (ER) is a critical organelle for cellular homeostasis, the mechanisms that mediate adaptation of the ER to metabolic challenges in adipocytes are unclear. Here we show that brown adipose tissue (BAT) thermogenic function requires an adaptive increase in proteasomal activity to secure cellular protein quality control, and we identify the ER-localized transcription factor nuclear factor erythroid 2-like 1 (Nfe2l1, also known as Nrf1) as a critical driver of this process. We show that cold adaptation induces Nrf1 in BAT to increase proteasomal activity and that this is crucial for maintaining ER homeostasis and cellular integrity, specifically when the cells are in a state of high thermogenic activity. In mice, under thermogenic conditions, brown-adipocyte-specific deletion of Nfe2l1 (Nrf1) resulted in ER stress, tissue inflammation, markedly diminished mitochondrial function and whitening of the BAT. In mouse models of both genetic and dietary obesity, stimulation of proteasomal activity by exogenously expressing Nrf1 or by treatment with the proteasome activator PA28α in BAT resulted in improved insulin sensitivity. In conclusion, Nrf1 emerges as a novel guardian of brown adipocyte function, providing increased proteometabolic quality control for adapting to cold or to obesity.
Conflict of interest statement
The authors declare no competing interest. The study was supported by an industry-sponsored research agreement between Harvard University and Servier.
Figures



Similar articles
-
Single-nucleus RNA-sequencing reveals NRF1/NFE2L1 as a key factor determining the thermogenesis and cellular heterogeneity and dynamics of brown adipose tissues in mice.Redox Biol. 2023 Nov;67:102879. doi: 10.1016/j.redox.2023.102879. Epub 2023 Sep 11. Redox Biol. 2023. PMID: 37716088 Free PMC article.
-
Adipocyte MTERF4 regulates non-shivering adaptive thermogenesis and sympathetic-dependent glucose homeostasis.Biochim Biophys Acta Mol Basis Dis. 2019 Jun 1;1865(6):1298-1312. doi: 10.1016/j.bbadis.2019.01.025. Epub 2019 Jan 26. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30690068
-
Overexpression of Adiponectin Receptor 1 Inhibits Brown and Beige Adipose Tissue Activity in Mice.Int J Mol Sci. 2021 Jan 18;22(2):906. doi: 10.3390/ijms22020906. Int J Mol Sci. 2021. PMID: 33477525 Free PMC article.
-
Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity.Biochimie. 2017 Mar;134:62-70. doi: 10.1016/j.biochi.2016.09.007. Epub 2016 Sep 10. Biochimie. 2017. PMID: 27621146 Review.
-
Energy dissipation in brown adipose tissue: from mice to men.Mol Cell Endocrinol. 2013 Oct 15;379(1-2):43-50. doi: 10.1016/j.mce.2013.04.017. Epub 2013 Apr 28. Mol Cell Endocrinol. 2013. PMID: 23632102 Review.
Cited by
-
The Heating Microenvironment: Intercellular Cross Talk Within Thermogenic Adipose Tissue.Diabetes. 2020 Aug;69(8):1599-1604. doi: 10.2337/db20-0303. Diabetes. 2020. PMID: 32690661 Free PMC article. Review.
-
CYC27 Synthetic Derivative of Bromophenol from Red Alga Rhodomela confervoides: Anti-Diabetic Effects of Sensitizing Insulin Signaling Pathways and Modulating RNA Splicing-Associated RBPs.Mar Drugs. 2019 Jan 11;17(1):49. doi: 10.3390/md17010049. Mar Drugs. 2019. PMID: 30641913 Free PMC article.
-
Myoglobin in Brown Adipose Tissue: A Multifaceted Player in Thermogenesis.Cells. 2023 Sep 8;12(18):2240. doi: 10.3390/cells12182240. Cells. 2023. PMID: 37759463 Free PMC article. Review.
-
Latest Advancements on Combating Obesity by Targeting Human Brown/Beige Adipose Tissues.Front Endocrinol (Lausanne). 2022 May 4;13:884944. doi: 10.3389/fendo.2022.884944. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35600577 Free PMC article. Review.
-
Shortwave infrared fluorescence imaging of peripheral organs in awake and freely moving mice.Front Neurosci. 2023 May 18;17:1135494. doi: 10.3389/fnins.2023.1135494. eCollection 2023. Front Neurosci. 2023. PMID: 37274204 Free PMC article.
References
Online methods references
-
- Rodbell M. Metabolism of Isolated Fat Cells. I. Effects of Hormones on Glucose Metabolism and Lipolysis. J Biol Chem. 1964;239:375–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

