Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model
- PMID: 29378639
- PMCID: PMC5789598
- DOI: 10.1186/s13287-018-0774-8
Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model
Abstract
Background: Mesenchymal stem (stromal) cells (MSCs) mediate their immunoregulatory and tissue repair functions by secreting paracrine factors, including extracellular vesicles (EVs). In several animal models of human diseases, MSC-EVs mimic the beneficial effects of MSCs. Influenza viruses cause annual outbreaks of acute respiratory illness resulting in significant mortality and morbidity. Influenza viruses constantly evolve, thus generating drug-resistant strains and rendering current vaccines less effective against the newly generated strains. Therefore, new therapies that can control virus replication and the inflammatory response of the host are needed. The objective of this study was to examine if MSC-EV treatment can attenuate influenza virus-induced acute lung injury in a preclinical model.
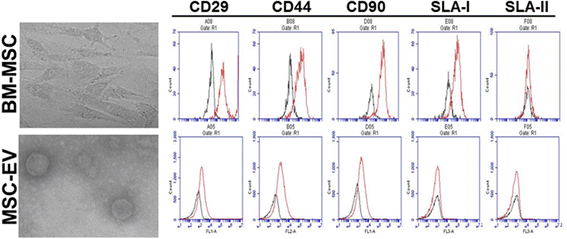
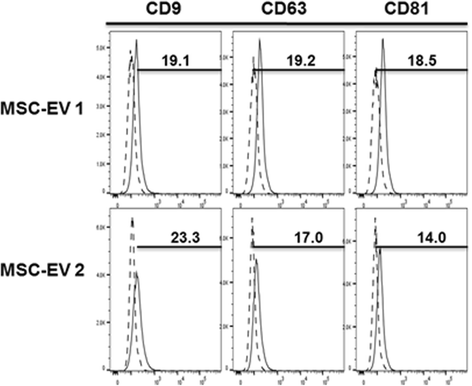
Methods: We isolated EVs from swine bone marrow-derived MSCs. Morphology of MSC-EVs was determined by electron microscopy and expression of mesenchymal markers was examined by flow cytometry. Next, we examined the anti-influenza activity of MSC-EVs in vitro in lung epithelial cells and anti-viral and immunomodulatory properties in vivo in a pig model of influenza virus.
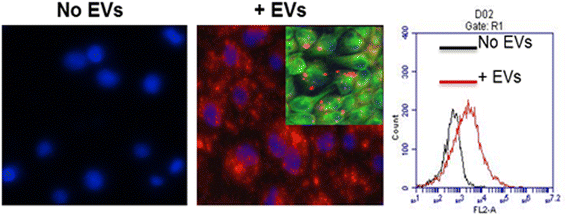
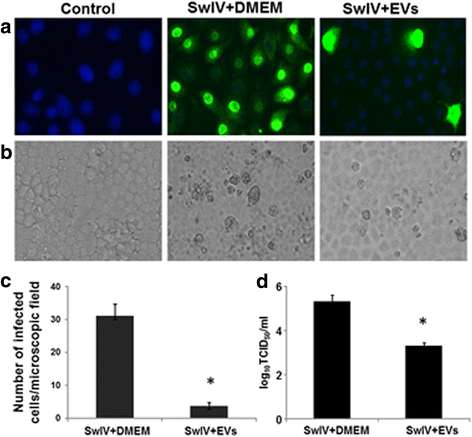
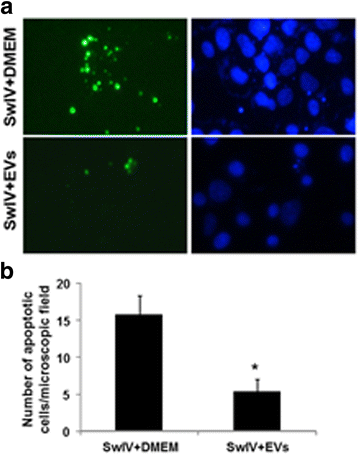
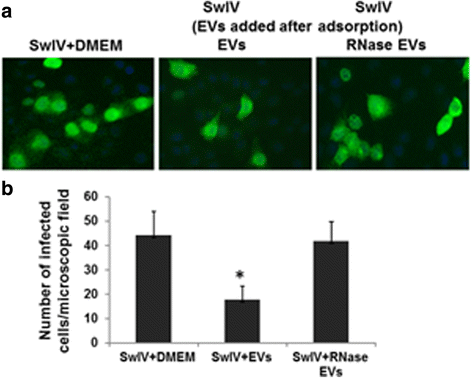
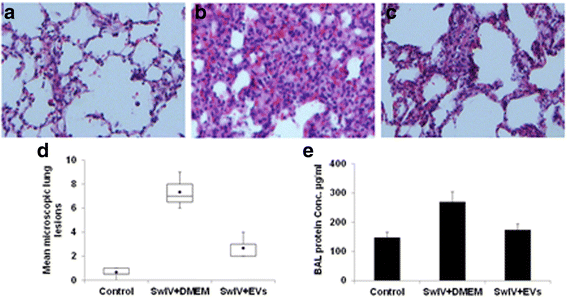
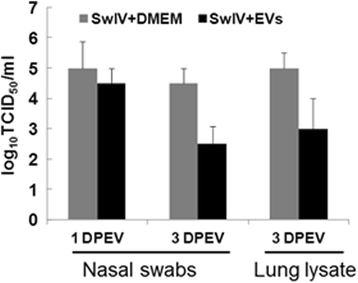
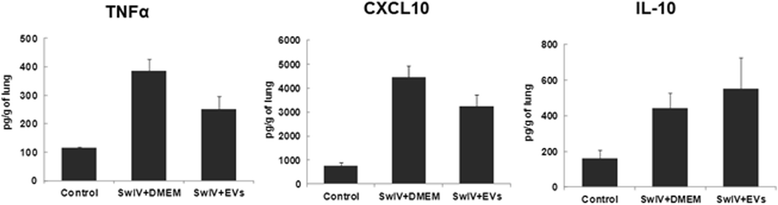
Results: MSC-EVs were isolated from MSC-conditioned medium by ultracentrifugation. MSC-EVs were round-shaped and, similarly to MSCs, expressed mesenchymal markers and lacked the expression of swine leukocyte antigens I and II. Incubation of PKH-26-labeled EVs with lung epithelial cells revealed that MSC-EVs incorporated into the epithelial cells. Next, we examined the anti-influenza and anti-inflammatory properties of MSC-EVs. MSC-EVs inhibited the hemagglutination activity of avian, swine, and human influenza viruses at concentrations of 1.25-5 μg/ml. MSC-EVs inhibited influenza virus replication and virus-induced apoptosis in lung epithelial cells. The anti-influenza activity of MSC-EVs was due to transfer of RNAs from EVs to epithelial cells since pre-incubation of MSC-EVs with RNase enzyme abrogated the anti-influenza activity of MSC-EVs. In a pig model of influenza virus, intratracheal administration of MSC-EVs 12 h after influenza virus infection significantly reduced virus shedding in the nasal swabs, influenza virus replication in the lungs, and virus-induced production of proinflammatory cytokines in the lungs of influenza-infected pigs. The histopathological findings revealed that MSC-EVs alleviated influenza virus-induced lung lesions in pigs.
Conclusions: Our data demonstrated in a relevant preclinical large animal model of influenza virus that MSC-EVs possessed anti-influenza and anti-inflammatory properties and that EVs may be used as cell-free therapy for influenza in humans.
Keywords: Acute lung injury; Extracellular vesicles; Influenza; Large animal model; Mesenchymal stem cells; Stem cell therapy.
Conflict of interest statement
Ethics approval and consent to participate
All experimental procedures involving pigs were conducted in accordance with the guidelines of the Institutional Laboratory Animal Care and Use Committee, The Ohio State University (protocol #2014A00000040).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles in SARS-CoV-2 and H1N1 influenza-induced acute lung injury.J Extracell Vesicles. 2024 Sep;13(9):e12495. doi: 10.1002/jev2.12495. J Extracell Vesicles. 2024. PMID: 39254228 Free PMC article.
-
Endothelial cell-derived extracellular vesicles modulate the therapeutic efficacy of mesenchymal stem cells through IDH2/TET pathway in ARDS.Cell Commun Signal. 2024 May 27;22(1):293. doi: 10.1186/s12964-024-01672-0. Cell Commun Signal. 2024. PMID: 38802896 Free PMC article.
-
Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma.J Trauma Acute Care Surg. 2018 Feb;84(2):245-256. doi: 10.1097/TA.0000000000001744. J Trauma Acute Care Surg. 2018. PMID: 29251710 Free PMC article.
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Cell-Free Therapy for Sepsis.Front Immunol. 2020 Apr 21;11:647. doi: 10.3389/fimmu.2020.00647. eCollection 2020. Front Immunol. 2020. PMID: 32373121 Free PMC article. Review.
Cited by
-
Regenerative Medicine in COVID-19 Treatment: Real Opportunities and Range of Promises.Stem Cell Rev Rep. 2021 Feb;17(1):163-175. doi: 10.1007/s12015-020-09994-5. Stem Cell Rev Rep. 2021. PMID: 32564256 Free PMC article. Review.
-
Translational Animal Models Provide Insight Into Mesenchymal Stromal Cell (MSC) Secretome Therapy.Front Cell Dev Biol. 2021 Mar 19;9:654885. doi: 10.3389/fcell.2021.654885. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33869217 Free PMC article. Review.
-
Nanomedicine therapies modulating Macrophage Dysfunction: a potential strategy to attenuate Cytokine Storms in severe infections.Theranostics. 2020 Jul 25;10(21):9591-9600. doi: 10.7150/thno.47982. eCollection 2020. Theranostics. 2020. PMID: 32863947 Free PMC article. Review.
-
Exosomal miRNAs in Lung Diseases: From Biologic Function to Therapeutic Targets.J Clin Med. 2019 Aug 29;8(9):1345. doi: 10.3390/jcm8091345. J Clin Med. 2019. PMID: 31470655 Free PMC article. Review.
-
Stem Cell-based therapies for COVID-19-related acute respiratory distress syndrome.J Cell Mol Med. 2022 May;26(9):2483-2504. doi: 10.1111/jcmm.17265. Epub 2022 Apr 14. J Cell Mol Med. 2022. PMID: 35426198 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

