Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood
- PMID: 29369434
- PMCID: PMC5908742
- DOI: 10.1111/aji.12816
Lipopolysaccharide-induced maternal inflammation induces direct placental injury without alteration in placental blood flow and induces a secondary fetal intestinal injury that persists into adulthood
Abstract
Problem: Premature birth complicates 10%-12% of deliveries. Infection and inflammation are the most common etiologies and are associated with increased offspring morbidity and mortality. We hypothesize that lipopolysaccharide (LPS)-induced maternal inflammation causes direct placenta injury and subsequent injury to the fetal intestine.
Method of study: Pregnant C57Bl6 mice were injected intraperitoneally on day 15.5 with 100 μg/kg LPS or saline. Maternal serum, amniotic fluid, placental samples, and ileal samples of offspring were obtained assessed for inflammation and/or injury. Maternal placental ultrasounds were performed. Placental DNA was isolated for microbiome analysis.
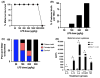
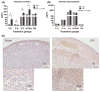
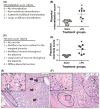
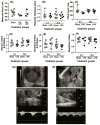
Results: Maternal injection with LPS caused elevated IL-1β, IL-10, IL-6, KC-GRO, and TNF. Placental tissue showed increased IL-1β, IL-6, and KC-GRO and decreased IL-10, but no changes were observed in amniotic fluid. Placental histology demonstrated LPS-induced increases in mineralization and necrosis, but no difference in placental blood flow. Most placentas had no detectable microbiome. Exposure to maternal LPS induced significant injury to the ilea of the offspring.
Conclusion: Lipopolysaccharide causes a maternal inflammatory response that is mirrored in the placenta. Placental histology demonstrates structural changes; however, placental blood flow is preserved. LPS also induces an indirect intestinal injury in the offspring that lasts beyond the neonatal period.
Keywords: cytokines; lipopolysaccharide; microbiome; mouse; placenta.
© 2018 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures


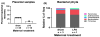
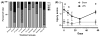
Similar articles
-
The fetal response to maternal inflammation is dependent upon maternal IL-6 in a murine model.Cytokine. 2023 Jul;167:156210. doi: 10.1016/j.cyto.2023.156210. Epub 2023 Apr 30. Cytokine. 2023. PMID: 37130487 Free PMC article.
-
Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury.Int J Dev Neurosci. 2011 Oct;29(6):663-71. doi: 10.1016/j.ijdevneu.2011.02.011. Epub 2011 Mar 4. Int J Dev Neurosci. 2011. PMID: 21382466 Free PMC article.
-
Mechanism of acute fetal cardiovascular depression after maternal inflammatory challenge in mouse.Am J Pathol. 2005 Jun;166(6):1585-92. doi: 10.1016/S0002-9440(10)62469-8. Am J Pathol. 2005. PMID: 15920144 Free PMC article.
-
Fetal and maternal vascular lesions.Semin Diagn Pathol. 2007 Feb;24(1):14-22. doi: 10.1053/j.semdp.2007.02.005. Semin Diagn Pathol. 2007. PMID: 17455858 Review.
-
Placental pathology in women with HIV.Placenta. 2021 Nov;115:27-36. doi: 10.1016/j.placenta.2021.09.006. Epub 2021 Sep 11. Placenta. 2021. PMID: 34537469 Review.
Cited by
-
Prenatal inflammation impairs intestinal microvascular development through a TNF-dependent mechanism and predisposes newborn mice to necrotizing enterocolitis.Am J Physiol Gastrointest Liver Physiol. 2019 Jul 1;317(1):G57-G66. doi: 10.1152/ajpgi.00332.2018. Epub 2019 May 24. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31125264 Free PMC article.
-
Moderately pathogenic maternal influenza A virus infection disrupts placental integrity but spares the fetal brain.Brain Behav Immun. 2021 Aug;96:28-39. doi: 10.1016/j.bbi.2021.05.004. Epub 2021 May 12. Brain Behav Immun. 2021. PMID: 33989741 Free PMC article.
-
Prenatal inflammation as a link between placental expression signature of tryptophan metabolism and preterm birth.Hum Mol Genet. 2021 Nov 1;30(22):2053-2067. doi: 10.1093/hmg/ddab169. Hum Mol Genet. 2021. PMID: 34169316 Free PMC article.
-
Maternal hepcidin determines embryo iron homeostasis in mice.Blood. 2020 Nov 5;136(19):2206-2216. doi: 10.1182/blood.2020005745. Blood. 2020. PMID: 32584957 Free PMC article.
-
Zinc-nanoparticles alleviate the ovarian damage induced by bacterial lipopolysaccharide (LPS) in pregnant rats and their fetuses.Histochem Cell Biol. 2023 Nov;160(5):453-475. doi: 10.1007/s00418-023-02222-4. Epub 2023 Jul 26. Histochem Cell Biol. 2023. PMID: 37495867 Free PMC article.
References
-
- Gabbe SG, Niebyl JR, Simpson JL. Obstetrics: Normal and Problem Pregnancies. 7. Philadelphia, PA: Elsevier/Saunders; 2017.
-
- Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore T, Greene MF. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice: Expert Consult Premium Edition - Enhanced Online Features. Houston, TX: Elsevier Health Sciences; 2013.
-
- Aziz N, Cheng YW, Caughey AB. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J Matern Fetal Neonatal Med. 2009;22:780–784. - PubMed
-
- Lau J, Magee F, Qiu Z, Hoube J, Von Dadelszen P, Lee SK. Chorioamnionitis with a fetal inflammatory response is associated with higher neonatal mortality, morbidity, and resource use than chorioamnionitis displaying a maternal inflammatory response only. Am J Obstet Gynecol. 2005;193:708–713. - PubMed
-
- Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

