The hypoxic tumour microenvironment
- PMID: 29362402
- PMCID: PMC5833859
- DOI: 10.1038/s41389-017-0011-9
The hypoxic tumour microenvironment
Abstract
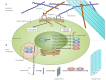
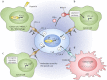
Cancer progression often benefits from the selective conditions present in the tumour microenvironment, such as the presence of cancer-associated fibroblasts (CAFs), deregulated ECM deposition, expanded vascularisation and repression of the immune response. Generation of a hypoxic environment and activation of its main effector, hypoxia-inducible factor-1 (HIF-1), are common features of advanced cancers. In addition to the impact on tumour cell biology, the influence that hypoxia exerts on the surrounding cells represents a critical step in the tumorigenic process. Hypoxia indeed enables a number of events in the tumour microenvironment that lead to the expansion of aggressive clones from heterogeneous tumour cells and promote a lethal phenotype. In this article, we review the most relevant findings describing the influence of hypoxia and the contribution of HIF activation on the major components of the tumour microenvironment, and we summarise their role in cancer development and progression.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Tumour response to hypoxia: understanding the hypoxic tumour microenvironment to improve treatment outcome in solid tumours.Front Oncol. 2024 Jan 30;14:1331355. doi: 10.3389/fonc.2024.1331355. eCollection 2024. Front Oncol. 2024. PMID: 38352889 Free PMC article. Review.
-
Cancer-Associated Fibroblasts in the Hypoxic Tumor Microenvironment.Cancers (Basel). 2022 Jul 7;14(14):3321. doi: 10.3390/cancers14143321. Cancers (Basel). 2022. PMID: 35884382 Free PMC article. Review.
-
HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs).Breast Cancer Res. 2013;15(4):R64. doi: 10.1186/bcr3458. Breast Cancer Res. 2013. PMID: 23947803 Free PMC article.
-
The IL1β-IL1R signaling is involved in the stimulatory effects triggered by hypoxia in breast cancer cells and cancer-associated fibroblasts (CAFs).J Exp Clin Cancer Res. 2020 Aug 10;39(1):153. doi: 10.1186/s13046-020-01667-y. J Exp Clin Cancer Res. 2020. PMID: 32778144 Free PMC article.
-
Decursin promotes HIF-1α proteasomal degradation and immune responses in hypoxic tumour microenvironment.Phytomedicine. 2020 Nov;78:153318. doi: 10.1016/j.phymed.2020.153318. Epub 2020 Sep 1. Phytomedicine. 2020. PMID: 32896707
Cited by
-
Adaptive Mechanisms of Tumor Therapy Resistance Driven by Tumor Microenvironment.Front Cell Dev Biol. 2021 Mar 1;9:641469. doi: 10.3389/fcell.2021.641469. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33732706 Free PMC article. Review.
-
Distinct Cargos of Small Extracellular Vesicles Derived from Hypoxic Cells and Their Effect on Cancer Cells.Int J Mol Sci. 2020 Jul 17;21(14):5071. doi: 10.3390/ijms21145071. Int J Mol Sci. 2020. PMID: 32709110 Free PMC article. Review.
-
Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect.Cell Biosci. 2021 Mar 20;11(1):56. doi: 10.1186/s13578-021-00570-z. Cell Biosci. 2021. PMID: 33743781 Free PMC article. Review.
-
Hypoxia-Nitric Oxide Axis and the Associated Damage Molecular Pattern in Cutaneous Melanoma.J Pers Med. 2022 Oct 4;12(10):1646. doi: 10.3390/jpm12101646. J Pers Med. 2022. PMID: 36294785 Free PMC article.
-
Mouse dendritic cells in the steady state: Hypoxia, autophagy, and stem cell factor.Cell Biochem Funct. 2022 Oct;40(7):718-728. doi: 10.1002/cbf.3737. Epub 2022 Sep 7. Cell Biochem Funct. 2022. PMID: 36069062 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

