The hematopoietic stem cell niche: from embryo to adult
- PMID: 29358215
- PMCID: PMC5825844
- DOI: 10.1242/dev.139691
The hematopoietic stem cell niche: from embryo to adult
Abstract
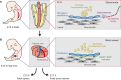
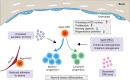
Hematopoietic stem cells (HSCs) develop in discrete anatomical niches, migrating during embryogenesis from the aorta-gonad-mesonephros (AGM) region to the fetal liver, and finally to the bone marrow, where most HSCs reside throughout adult life. These niches provide supportive microenvironments that specify, expand and maintain HSCs. Understanding the constituents and molecular regulation of HSC niches is of considerable importance as it could shed new light on the mechanistic principles of HSC emergence and maintenance, and provide novel strategies for regenerative medicine. However, controversy exists concerning the cellular complexity of the bone marrow niche, and our understanding of the different HSC niches during development remains limited. In this Review, we summarize and discuss what is known about the heterogeneity of the HSC niches at distinct stages of their ontogeny, from the embryo to the adult bone marrow, drawing predominantly on data from mouse studies.
Keywords: Bone marrow; Hematopoietic stem cell; Hemogenic endothelium; Microenvironment; Niche.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells.J Clin Invest. 2015 May;125(5):2032-45. doi: 10.1172/JCI80137. Epub 2015 Apr 13. J Clin Invest. 2015. PMID: 25866967 Free PMC article.
-
In vivo differentiation of stem cells in the aorta-gonad-mesonephros region of mouse embryo and adult bone marrow.Exp Hematol. 2002 Aug;30(8):957-66. doi: 10.1016/s0301-472x(02)00822-6. Exp Hematol. 2002. PMID: 12160848
-
[AGM region and hematopoiesis during ontogeny--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005 Feb;13(1):164-8. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005. PMID: 15748460 Review. Chinese.
-
Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity.Blood. 2002 Feb 15;99(4):1183-9. doi: 10.1182/blood.v99.4.1183. Blood. 2002. PMID: 11830464
-
Role of the microenvironment of the embryonic aorta-gonad-mesonephros region in hematopoiesis.Ann N Y Acad Sci. 2001 Jun;938:109-16. doi: 10.1111/j.1749-6632.2001.tb03579.x. Ann N Y Acad Sci. 2001. PMID: 11458497 Review.
Cited by
-
WNT5A from the fetal liver vascular niche supports human fetal liver hematopoiesis.Stem Cell Res Ther. 2021 Jun 5;12(1):321. doi: 10.1186/s13287-021-02380-z. Stem Cell Res Ther. 2021. PMID: 34090485 Free PMC article.
-
Neurogenic Heterotopic Ossifications Recapitulate Hematopoietic Stem Cell Niche Development Within an Adult Osteogenic Muscle Environment.Front Cell Dev Biol. 2021 Mar 5;9:611842. doi: 10.3389/fcell.2021.611842. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33748104 Free PMC article. Review.
-
Liposomes trigger bone marrow niche macrophage "foam" cell formation and affect hematopoiesis in mice.J Lipid Res. 2022 Oct;63(10):100273. doi: 10.1016/j.jlr.2022.100273. Epub 2022 Sep 7. J Lipid Res. 2022. PMID: 36084713 Free PMC article.
-
MIF/CXCR4 signaling axis contributes to survival, invasion, and drug resistance of metastatic neuroblastoma cells in the bone marrow microenvironment.BMC Cancer. 2022 Jun 17;22(1):669. doi: 10.1186/s12885-022-09725-8. BMC Cancer. 2022. PMID: 35715791 Free PMC article.
-
Conditionally pathogenic genetic variants of a hematopoietic disease-suppressing enhancer.Sci Adv. 2021 Dec 10;7(50):eabk3521. doi: 10.1126/sciadv.abk3521. Epub 2021 Dec 10. Sci Adv. 2021. PMID: 34890222 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

