Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma
- PMID: 29353553
- PMCID: PMC5776777
- DOI: 10.1186/s40425-018-0315-0
Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma
Abstract
Background: An elevated Neutrophil-to-lymphocyte ratio (NLR) is associated with worse outcomes in several malignancies. However, its role with contemporary immune checkpoint blockade (ICB) is unknown. We investigated the utility of NLR in metastatic renal cell carcinoma (mRCC) patients treated with PD-1/PD-L1 ICB.
Methods: We examined NLR at baseline and 6 (±2) weeks later in 142 patients treated between 2009 and 2017 at Dana-Farber Cancer Institute (Boston, USA). Landmark analysis at 6 weeks was conducted to explore the prognostic value of relative NLR change on overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). Cox and logistic regression models allowed for adjustment of line of therapy, number of IMDC risk factors, histology and baseline NLR.
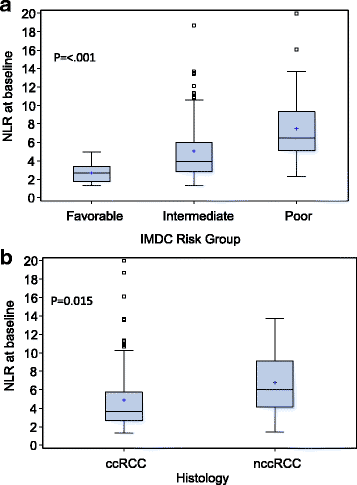
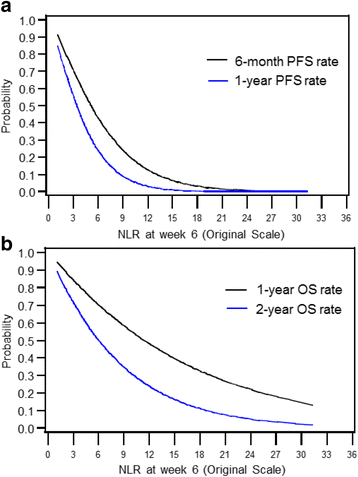
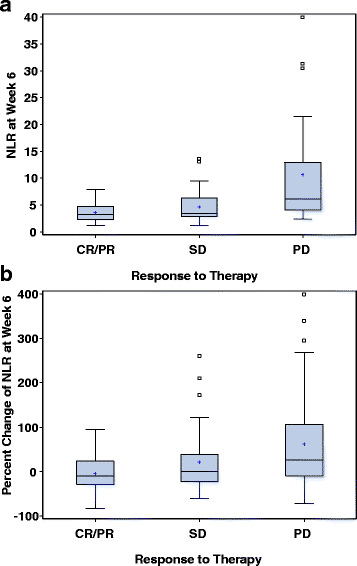
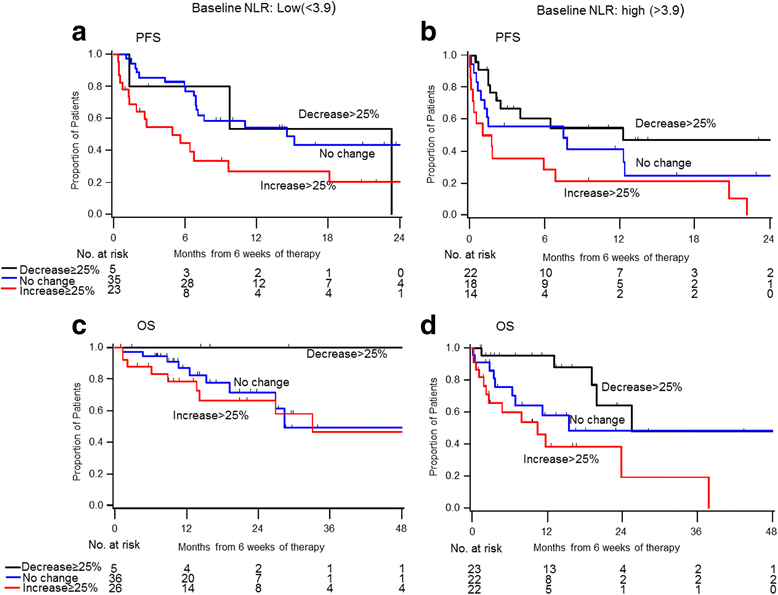
Results: Median follow up was 16.6 months (range: 0.7-67.8). Median duration on therapy was 5.1 months (<1-61.4). IMDC risk groups were: 18% favorable, 60% intermediate, 23% poor-risk. Forty-four percent were on first-line ICB and 56% on 2nd line or more. Median NLR was 3.9 (1.3-42.4) at baseline and 4.1 (1.1-96.4) at week 6. Patients with a higher baseline NLR showed a trend toward lower ORR, shorter PFS, and shorter OS. Higher NLR at 6 weeks was a significantly stronger predictor of all three outcomes than baseline NLR. Relative NLR change by ≥25% from baseline to 6 weeks after ICB therapy was associated with reduced ORR and an independent prognostic factor for PFS (p < 0.001) and OS (p = 0.004), whereas a decrease in NLR by ≥25% was associated with improved outcomes.
Conclusions: Early decline and NLR at 6 weeks are associated with significantly improved outcomes in mRCC patients treated with ICB. The prognostic value of the readily-available NLR warrants larger, prospective validation.
Keywords: Immunotherapy; Neutrophil-to-lymphocyte ratio; PD-1/pd-L1 PD-L1; Prognostic biomarker; Renal cell carcinoma.
Conflict of interest statement
Authors information
Not applicable.
Ethics approval and consent to participate
The study was approved by the local institutional review boards and was conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helskinki.
Consent for publication
Informed consent for publication has been obtained and the consent forms are held by the authors.
Competing interests
The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
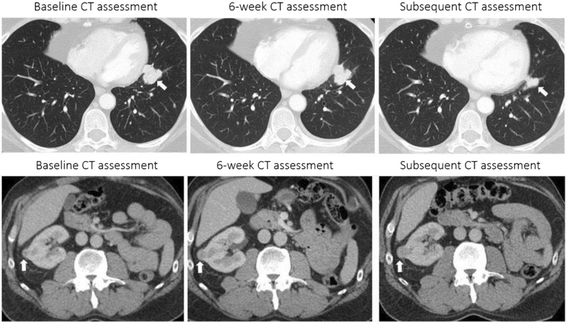
Figures
Similar articles
-
Change in Neutrophil-to-lymphocyte Ratio in Response to Targeted Therapy for Metastatic Renal Cell Carcinoma as a Prognosticator and Biomarker of Efficacy.Eur Urol. 2016 Aug;70(2):358-64. doi: 10.1016/j.eururo.2016.02.033. Epub 2016 Feb 28. Eur Urol. 2016. PMID: 26924770 Clinical Trial.
-
Checkpoint inhibitors in patients with metastatic renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium.Cancer. 2018 Sep 15;124(18):3677-3683. doi: 10.1002/cncr.31595. Epub 2018 Oct 11. Cancer. 2018. PMID: 30307610
-
Neutrophil-lymphocyte ratio as a predictive biomarker for response to high dose interleukin-2 in patients with renal cell carcinoma.BMC Urol. 2017 Jan 5;17(1):1. doi: 10.1186/s12894-016-0192-0. BMC Urol. 2017. PMID: 28056941 Free PMC article.
-
Sequencing and Combination of Systemic Therapy in Metastatic Renal Cell Carcinoma.Eur Urol Oncol. 2019 Sep;2(5):505-514. doi: 10.1016/j.euo.2019.06.022. Epub 2019 Aug 1. Eur Urol Oncol. 2019. PMID: 31377308 Review.
-
A Critical Insight into the Clinical Translation of PD-1/PD-L1 Blockade Therapy in Clear Cell Renal Cell Carcinoma.Curr Urol Rep. 2019 Jan 15;20(1):1. doi: 10.1007/s11934-019-0866-8. Curr Urol Rep. 2019. PMID: 30645700 Review.
Cited by
-
Tyrosine Kinase Inhibitor Cabozantinib Inhibits Murine Renal Cancer by Activating Innate and Adaptive Immunity.Front Oncol. 2021 Apr 19;11:663517. doi: 10.3389/fonc.2021.663517. eCollection 2021. Front Oncol. 2021. PMID: 33954115 Free PMC article.
-
Prognostic value of the postoperative neutrophil-lymphocyte ratio in solid tumors: A meta-analysis.PLoS One. 2021 Apr 19;16(4):e0250091. doi: 10.1371/journal.pone.0250091. eCollection 2021. PLoS One. 2021. PMID: 33872342 Free PMC article.
-
Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma.Nat Rev Urol. 2023 Mar;20(3):133-157. doi: 10.1038/s41585-022-00676-0. Epub 2022 Nov 21. Nat Rev Urol. 2023. PMID: 36414800 Review.
-
Predicting Response to Immunotherapy in Metastatic Renal Cell Carcinoma.Cancers (Basel). 2020 Sep 18;12(9):2662. doi: 10.3390/cancers12092662. Cancers (Basel). 2020. PMID: 32961934 Free PMC article. Review.
-
The lymphocyte-to-monocyte ratio could predict the efficacy of PD-1 inhibitors in patients with advanced cancer.Transl Cancer Res. 2020 Jul;9(7):4111-4120. doi: 10.21037/tcr-20-1451. Transl Cancer Res. 2020. PMID: 35117780 Free PMC article.
References
-
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
