Protein neddylation and its alterations in human cancers for targeted therapy
- PMID: 29331584
- PMCID: PMC5829022
- DOI: 10.1016/j.cellsig.2018.01.009
Protein neddylation and its alterations in human cancers for targeted therapy
Abstract
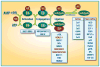
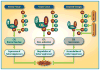
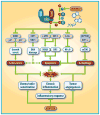
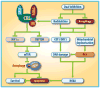
Neddylation, a post-translational modification that conjugates an ubiquitin-like protein NEDD8 to substrate proteins, is an important biochemical process that regulates protein function. The best-characterized substrates of neddylation are the cullin subunits of Cullin-RING ligases (CRLs), which, as the largest family of E3 ubiquitin ligases, control many important biological processes, including tumorigenesis, through promoting ubiquitylation and subsequent degradation of a variety of key regulatory proteins. Recently, increasing pieces of experimental evidence strongly indicate that the process of protein neddylation modification is elevated in multiple human cancers, providing sound rationale for its targeting as an attractive anticancer therapeutic strategy. Indeed, neddylation inactivation by MLN4924 (also known as pevonedistat), a small molecule inhibitor of E1 NEDD8-activating enzyme currently in phase I/II clinical trials, exerts significant anticancer effects by inducing cell cycle arrest, apoptosis, senescence and autophagy in a cell-type and context dependent manner. Here, we summarize the latest progresses in the field with a major focus on preclinical studies in validation of neddylation modification as a promising anticancer target.
Keywords: Apoptosis; Autophagy; Cancer target; Cell cycle arrest; Cullin RING ligase; MLN4924; Neddylation; Senescence.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Targeting Protein Neddylation for Cancer Therapy.Adv Exp Med Biol. 2020;1217:297-315. doi: 10.1007/978-981-15-1025-0_18. Adv Exp Med Biol. 2020. PMID: 31898235 Review.
-
Suppression of tumor angiogenesis by targeting the protein neddylation pathway.Cell Death Dis. 2014 Feb 13;5(2):e1059. doi: 10.1038/cddis.2014.21. Cell Death Dis. 2014. PMID: 24525735 Free PMC article.
-
Neddylation inhibitor MLN4924 suppresses growth and migration of human gastric cancer cells.Sci Rep. 2016 Apr 11;6:24218. doi: 10.1038/srep24218. Sci Rep. 2016. PMID: 27063292 Free PMC article.
-
A first-in-class inhibitor, MLN4924 (pevonedistat), induces cell-cycle arrest, senescence, and apoptosis in human renal cell carcinoma by suppressing UBE2M-dependent neddylation modification.Cancer Chemother Pharmacol. 2018 Jun;81(6):1083-1093. doi: 10.1007/s00280-018-3582-z. Epub 2018 Apr 17. Cancer Chemother Pharmacol. 2018. PMID: 29667067
-
Targeting Neddylation pathways to inactivate cullin-RING ligases for anticancer therapy.Antioxid Redox Signal. 2014 Dec 10;21(17):2383-400. doi: 10.1089/ars.2013.5795. Epub 2014 Feb 20. Antioxid Redox Signal. 2014. PMID: 24410571 Free PMC article. Review.
Cited by
-
Overexpressed NEDD8 as a potential therapeutic target in esophageal squamous cell carcinoma.Cancer Biol Med. 2021 Mar 18;19(4):504-17. doi: 10.20892/j.issn.2095-3941.2020.0484. Online ahead of print. Cancer Biol Med. 2021. PMID: 33733647 Free PMC article.
-
Camptothecin Inhibits Neddylation to Activate the Protective Autophagy Through NF-κB/AMPK/mTOR/ULK1 Axis in Human Esophageal Cancer Cells.Front Oncol. 2021 Apr 8;11:671180. doi: 10.3389/fonc.2021.671180. eCollection 2021. Front Oncol. 2021. PMID: 33898327 Free PMC article.
-
NEDD8-conjugating enzyme E2s: critical targets for cancer therapy.Cell Death Discov. 2023 Jan 23;9(1):23. doi: 10.1038/s41420-023-01337-w. Cell Death Discov. 2023. PMID: 36690633 Free PMC article. Review.
-
UBE2M Drives Hepatocellular Cancer Progression as a p53 Negative Regulator by Binding to MDM2 and Ribosomal Protein L11.Cancers (Basel). 2021 Sep 29;13(19):4901. doi: 10.3390/cancers13194901. Cancers (Basel). 2021. PMID: 34638383 Free PMC article.
-
Deubiquitinating enzymes (DUBs): DoUBle-edged swords in CNS autoimmunity.J Neuroinflammation. 2020 Apr 6;17(1):102. doi: 10.1186/s12974-020-01783-8. J Neuroinflammation. 2020. PMID: 32248814 Free PMC article. Review.
References
-
- Kamitani T, Kito K, Nguyen HP, Yeh ET. Characterization of NEDD8, a developmentally down-regulated ubiquitin-like protein. J Biol Chem. 1997;272(45):28557–62. - PubMed
-
- Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;36(Pt 5):802–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

