Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study
- PMID: 29329425
- PMCID: PMC5888952
- DOI: 10.1093/toxsci/kfx289
Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study
Abstract
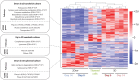
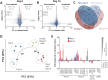
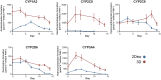
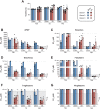
Primary human hepatocytes (PHHs) are commonly used for in vitro studies of drug-induced liver injury. However, when cultured as 2D monolayers, PHH lose crucial hepatic functions within hours. This dedifferentiation can be ameliorated when PHHs are cultured in sandwich configuration (2Dsw), particularly when cultures are regularly re-overlaid with extracellular matrix, or as 3D spheroids. In this study, the 6 participating laboratories evaluated the robustness of these 2 model systems made from cryopreserved PHH from the same donors considering both inter-donor and inter-laboratory variability and compared their suitability for use in repeated-dose toxicity studies using 5 different hepatotoxins with different toxicity mechanisms. We found that expression levels of proteins involved in drug absorption, distribution, metabolism, and excretion, as well as catalytic activities of 5 different CYPs, were significantly higher in 3D spheroid cultures, potentially affecting the exposure of the cells to drugs and their metabolites. Furthermore, global proteomic analyses revealed that PHH in 3D spheroid configuration were temporally stable whereas proteomes from the same donors in 2Dsw cultures showed substantial alterations in protein expression patterns over the 14 days in culture. Overall, spheroid cultures were more sensitive to the hepatotoxic compounds investigated, particularly upon long-term exposures, across testing sites with little inter-laboratory or inter-donor variability. The data presented here suggest that repeated-dosing regimens improve the predictivity of in vitro toxicity assays, and that PHH spheroids provide a sensitive and robust system for long-term mechanistic studies of drug-induced hepatotoxicity, whereas the 2Dsw system has a more dedifferentiated phenotype and lower sensitivity to detect hepatotoxicity.
Figures

Similar articles
-
Inter-individual differences in the susceptibility of primary human hepatocytes towards drug-induced cholestasis are compound and time dependent.Toxicol Lett. 2018 Oct 1;295:187-194. doi: 10.1016/j.toxlet.2018.06.1069. Epub 2018 Jun 18. Toxicol Lett. 2018. PMID: 29913214
-
Prediction of Drug-Induced Hepatotoxicity Using Long-Term Stable Primary Hepatic 3D Spheroid Cultures in Chemically Defined Conditions.Toxicol Sci. 2018 Jun 1;163(2):655-665. doi: 10.1093/toxsci/kfy058. Toxicol Sci. 2018. PMID: 29590495 Free PMC article.
-
Three-Dimensional Spheroid Primary Human Hepatocytes in Monoculture and Coculture with Nonparenchymal Cells.Tissue Eng Part C Methods. 2018 Sep;24(9):534-545. doi: 10.1089/ten.TEC.2018.0134. Tissue Eng Part C Methods. 2018. PMID: 30101670
-
Evolution of Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates.Basic Clin Pharmacol Toxicol. 2017 Oct;121(4):234-238. doi: 10.1111/bcpt.12804. Epub 2017 Jul 3. Basic Clin Pharmacol Toxicol. 2017. PMID: 28470941 Review.
-
Three-Dimensional Liver Culture Systems to Maintain Primary Hepatic Properties for Toxicological Analysis In Vitro.Int J Mol Sci. 2021 Sep 23;22(19):10214. doi: 10.3390/ijms221910214. Int J Mol Sci. 2021. PMID: 34638555 Free PMC article. Review.
Cited by
-
Adaptation of the in vitro micronucleus assay for genotoxicity testing using 3D liver models supporting longer-term exposure durations.Mutagenesis. 2020 Sep 12;35(4):319-330. doi: 10.1093/mutage/geaa018. Mutagenesis. 2020. PMID: 32780103 Free PMC article.
-
Analysis of reproducibility and robustness of OrganoPlate® 2-lane 96, a liver microphysiological system for studies of pharmacokinetics and toxicological assessment of drugs.Toxicol In Vitro. 2022 Dec;85:105464. doi: 10.1016/j.tiv.2022.105464. Epub 2022 Aug 31. Toxicol In Vitro. 2022. PMID: 36057418 Free PMC article.
-
A Review of CYP-Mediated Drug Interactions: Mechanisms and In Vitro Drug-Drug Interaction Assessment.Biomolecules. 2024 Jan 12;14(1):99. doi: 10.3390/biom14010099. Biomolecules. 2024. PMID: 38254699 Free PMC article. Review.
-
Three-dimensional HepaRG spheroids as a liver model to study human genotoxicity in vitro with the single cell gel electrophoresis assay.Sci Rep. 2019 Jul 22;9(1):10548. doi: 10.1038/s41598-019-47114-7. Sci Rep. 2019. PMID: 31332230 Free PMC article.
-
Hepatocyte Thorns, A Novel Drug-Induced Stress Response in Human and Mouse Liver Spheroids.Cells. 2022 May 10;11(10):1597. doi: 10.3390/cells11101597. Cells. 2022. PMID: 35626634 Free PMC article.
References
-
- Bell C. C., Hendriks D. F. G., Moro S. M. L., Ellis E., Walsh J., Renblom A., Fredriksson Puigvert L., Dankers A. C. A., Jacobs F., Snoeys J. et al. , (2016). Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Scientific Reports 6, 25187.. - PMC - PubMed
-
- Bell C. C., Lauschke V. M., Vorrink S. U., Palmgren H., Duffin R., Andersson T. B., Ingelman-Sundberg M. (2017). Transcriptional, functional, and mechanistic comparisons of stem cell-derived hepatocytes, HepaRG cells, and three-dimensional human hepatocyte spheroids as predictive in vitro systems for drug-induced liver injury. Drug Metabolism and Disposition 45, 419–429. - PMC - PubMed
-
- Bellwon P., Truisi G. L., Bois F. Y., Wilmes A., Schmidt T., Savary C. C., Parmentier C., Hewitt P. G., Schmal O., Josse R. et al. , (2015). Kinetics and dynamics of cyclosporine A in three hepatic cell culture systems. Toxicology in Vitro 30, 62–78. - PubMed
-
- Bort R., Macé K., Boobis A., Gómez-Lechón M. J., Pfeifer A., Castell J. (1999). Hepatic metabolism of diclofenac: Role of human CYP in the minor oxidative pathways. Biochemical Pharmacology 58, 787–796. - PubMed
-
- Bowsher R. R., Compton J. A., Kirkwood J. A., Place G. D., Jones C. D., Mabry T. E., Hyslop D. L., Hatcher B. L., DeSante K. A. (1994). Sensitive and specific radioimmunoassay for fialuridine: Initial assessment of pharmacokinetics after single oral doses to healthy volunteers. Antimicrobial Agents and Chemotherapy 38, 2134–2142. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

