Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer
- PMID: 29326689
- PMCID: PMC5741703
- DOI: 10.3389/fimmu.2017.01770
Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer
Abstract
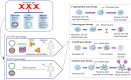
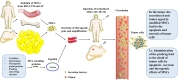
In recent years, in light of the promising potentials of mesenchymal stromal/stem cells (MSCs) for carrying therapeutic anticancer genes, a complete revisitation on old chemotherapy-based paradigms has been established. This review attempted to bring forward and introduce the novel therapeutic opportunities of using genetically engineered MSCs. The simplicities and advantages of MSCs for medical applications make them a unique and promising option in the case of cancer therapy. Some of the superiorities of using MSCs as therapeutic gene micro-carriers are the easy cell-extraction procedures and their abundant proliferation capacity in vitro without losing their main biological properties. Targeted therapy by using MSCs as the delivery vehicles of therapeutic genes is a new approach in the treatment of various types of cancers. Some of the distinct properties of MSCs, such as tumor-tropism, non-immunogenicity, stimulatory effect on the anti-inflammatory molecules, inhibitory effect on inflammatory responses, non-toxicity against the normal tissues, and easy processes for the clinical use, have formed the basis of attention to MSCs. They can be easily used for the treatment of damaged or injured tissues, regenerative medicine, and immune disorders. This review focused on the drugability of MSCs and their potential for the delivery of candidate anticancer genes. It also briefly reviewed the vectors and methods used for MSC-mediated gene therapy of malignancies. Also, the challenges, limitations, and considerations in using MSCs for gene therapy of cancer and the new methods developed for resolution of these problems are reviewed.
Keywords: cancer; cell therapy; gene therapy; mesenchymal stem cells; vector.
Figures

Similar articles
-
Inhibitory effect of genetically engineered mesenchymal stem cells with Apoptin on hepatoma cells in vitro and in vivo.Mol Cell Biochem. 2016 May;416(1-2):193-203. doi: 10.1007/s11010-016-2707-0. Epub 2016 May 3. Mol Cell Biochem. 2016. PMID: 27142531
-
Mesenchymal stem cells as the game-changing tools in the treatment of various organs disorders: Mirage or reality?J Cell Physiol. 2019 Feb;234(2):1268-1288. doi: 10.1002/jcp.27152. Epub 2018 Sep 7. J Cell Physiol. 2019. PMID: 30191962 Review.
-
Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche.Methods. 2016 Apr 15;99:62-8. doi: 10.1016/j.ymeth.2015.09.016. Epub 2015 Sep 15. Methods. 2016. PMID: 26384580 Review.
-
Genetically engineered mesenchymal stem cell therapy using self-assembling supramolecular hydrogels.J Control Release. 2015 Dec 28;220(Pt A):119-129. doi: 10.1016/j.jconrel.2015.10.034. Epub 2015 Oct 17. J Control Release. 2015. PMID: 26485045 Review.
-
Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas.Eur J Cancer. 2012 Jan;48(1):129-37. doi: 10.1016/j.ejca.2011.04.033. Epub 2011 Jun 12. Eur J Cancer. 2012. PMID: 21664124
Cited by
-
Dose-effect relationship and molecular mechanism by which BMSC-derived exosomes promote peripheral nerve regeneration after crush injury.Stem Cell Res Ther. 2020 Aug 18;11(1):360. doi: 10.1186/s13287-020-01872-8. Stem Cell Res Ther. 2020. PMID: 32811548 Free PMC article.
-
Current Strategies to Enhance Adipose Stem Cell Function: An Update.Int J Mol Sci. 2019 Aug 5;20(15):3827. doi: 10.3390/ijms20153827. Int J Mol Sci. 2019. PMID: 31387282 Free PMC article. Review.
-
Scalable manufacturing of gene-modified human mesenchymal stromal cells with microcarriers in spinner flasks.Appl Microbiol Biotechnol. 2023 Sep;107(18):5669-5685. doi: 10.1007/s00253-023-12634-w. Epub 2023 Jul 20. Appl Microbiol Biotechnol. 2023. PMID: 37470820 Free PMC article.
-
The Therapeutic Effects of Mesenchymal Stem Cells and their Secretome on Oral Squamous Cell Carcinoma.Curr Mol Med. 2024;24(10):1195-1207. doi: 10.2174/1566524023666230627151809. Curr Mol Med. 2024. PMID: 37366360 Review.
-
Need for Specialized Therapeutic Stem Cells Banks Equipped with Tumor Regression Enzymes and Anti-Tumor Genes.J Biomed Allied Res. 2020 Jun;2(1):013. doi: 10.37191/mapsci-2582-4937-2(1)-013. Epub 2020 Mar 16. J Biomed Allied Res. 2020. PMID: 33554055 Free PMC article.
References
-
- Walther W. Current Strategies in Cancer Gene Therapy. Switzerland: Springer International Publishing; (2016).
-
- Esmaeilzadeh A, Farshbaf A. Mesenchymal stem cell as a vector for gene and cell therapy strategies. Global J Stem Cell Biol Transplant (2015) 1(1):17–8.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

