Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo
- PMID: 29317369
- PMCID: PMC5831499
- DOI: 10.1016/j.actbio.2017.12.043
Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo
Abstract
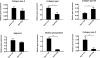
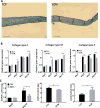
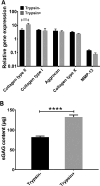
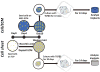
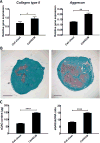
Mesenchymal stem cell derived extracellular matrix (MSC-ECM) is a natural biomaterial with robust bioactivity and good biocompatibility, and has been studied as a scaffold for tissue engineering. In this investigation, we tested the applicability of using decellularized human bone marrow derived MSC-ECM (hBMSC-ECM) as a culture substrate for chondrocyte expansion in vitro, as well as a scaffold for chondrocyte-based cartilage repair. hBMSC-ECM deposited by hBMSCs cultured on tissue culture plastic (TCP) was harvested, and then subjected to a decellularization process to remove hBMSCs. Compared with chondrocytes grown on TCP, chondrocytes seeded onto hBMSC-ECM exhibited significantly increased proliferation rate, and maintained better chondrocytic phenotype than TCP group. After being expanded to the same cell number and placed in high-density micromass cultures, chondrocytes from the ECM group showed better chondrogenic differentiation profile than those from the TCP group. To test cartilage formation ability, composites of hBMSC-ECM impregnated with chondrocytes were subjected to brief trypsin treatment to allow cell-mediated contraction, and folded to form 3-dimensional chondrocyte-impregnated hBMSC-ECM (Cell/ECM constructs). Upon culture in vitro in chondrogenic medium for 21 days, robust cartilage formation was observed in the Cell/ECM constructs. Similarly prepared Cell/ECM constructs were tested in vivo by subcutaneous implantation into SCID mice. Prominent cartilage formation was observed in the implanted Cell/ECM constructs 14 days post-implantation, with higher sGAG deposition compared to controls consisting of chondrocyte cell sheets. Taken together, these findings demonstrate that hBMSC-ECM is a superior culture substrate for chondrocyte expansion and a bioactive matrix potentially applicable for cartilage regeneration in vivo.
Statement of significance: Current cell-based treatments for focal cartilage defects face challenges, including chondrocyte dedifferentiation, need for xenogenic scaffolds, and suboptimal cartilage formation. We present here a novel technique that utilizes adult stem cell-derived extracellular matrix, as a culture substrate and/or encapsulation scaffold for human adult chondrocytes, for the repair of cartilage defects. Chondrocytes cultured in stem cell-derived matrix showed higher proliferation, better chondrocytic phenotype, and improved redifferentiation ability upon in vitro culture expansion. Most importantly, 3-dimensional constructs formed from chondrocytes folded within stem cell matrix manifested excellent cartilage formation both in vitro and in vivo. These findings demonstrate the suitability of stem cell-derived extracellular matrix as a culture substrate for chondrocyte expansion as well as a candidate bioactive matrix for cartilage regeneration.
Keywords: Bone marrow mesenchymal stem cells; Chondrocyte expansion; Chondrogenesis; Extracellular matrix; In vivo cartilage formation; Micromass; Redifferentiation.
Copyright © 2018 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



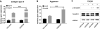
Similar articles
-
Extracellular matrix derived from chondrocytes promotes rapid expansion of human primary chondrocytes in vitro with reduced dedifferentiation.Acta Biomater. 2019 Feb;85:75-83. doi: 10.1016/j.actbio.2018.12.006. Epub 2018 Dec 5. Acta Biomater. 2019. PMID: 30528605
-
Extracellular matrix derived by human umbilical cord-deposited mesenchymal stem cells accelerates chondrocyte proliferation and differentiation potential in vitro.Cell Tissue Bank. 2019 Sep;20(3):351-365. doi: 10.1007/s10561-019-09774-7. Epub 2019 Jun 19. Cell Tissue Bank. 2019. PMID: 31218457
-
Cartilaginous extracellular matrix derived from decellularized chondrocyte sheets for the reconstruction of osteochondral defects in rabbits.Acta Biomater. 2018 Nov;81:129-145. doi: 10.1016/j.actbio.2018.10.005. Epub 2018 Oct 6. Acta Biomater. 2018. PMID: 30300711
-
The Challenge in Using Mesenchymal Stromal Cells for Recellularization of Decellularized Cartilage.Stem Cell Rev Rep. 2017 Feb;13(1):50-67. doi: 10.1007/s12015-016-9699-8. Stem Cell Rev Rep. 2017. PMID: 27826794 Review.
-
Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells.Tissue Eng Part B Rev. 2014 Dec;20(6):641-54. doi: 10.1089/ten.TEB.2014.0034. Epub 2014 Jun 23. Tissue Eng Part B Rev. 2014. PMID: 24834484 Free PMC article. Review.
Cited by
-
Advances in cartilage tissue regeneration: a review of stem cell therapies, tissue engineering, biomaterials, and clinical trials.EXCLI J. 2024 Sep 3;23:1170-1182. doi: 10.17179/excli2024-7088. eCollection 2024. EXCLI J. 2024. PMID: 39391058 Free PMC article. Review.
-
Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair.Bioact Mater. 2020 Sep 18;6(3):589-601. doi: 10.1016/j.bioactmat.2020.09.003. eCollection 2021 Mar. Bioact Mater. 2020. PMID: 33005824 Free PMC article.
-
Compositional and structural analysis of glycosaminoglycans in cell-derived extracellular matrices.Glycoconj J. 2019 Apr;36(2):141-154. doi: 10.1007/s10719-019-09858-2. Epub 2019 Jan 14. Glycoconj J. 2019. PMID: 30637588 Free PMC article.
-
Evaluation of genetic response of mesenchymal stem cells to nanosecond pulsed electric fields by whole transcriptome sequencing.World J Stem Cells. 2024 Mar 26;16(3):305-323. doi: 10.4252/wjsc.v16.i3.305. World J Stem Cells. 2024. PMID: 38577234 Free PMC article.
-
Polysaccharide-based hydrogels for cartilage regeneration.Front Cell Dev Biol. 2024 Oct 11;12:1444358. doi: 10.3389/fcell.2024.1444358. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39463764 Free PMC article. Review.
References
-
- Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–182. - PubMed
-
- Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211–215. - PubMed
-
- Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, Arøen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231–237. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

