Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress
- PMID: 29315210
- PMCID: PMC5793066
- DOI: 10.3390/md16010018
Isoaaptamine Induces T-47D Cells Apoptosis and Autophagy via Oxidative Stress
Abstract
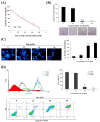
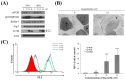
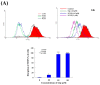
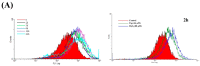
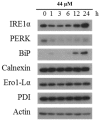
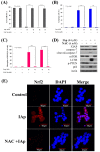
Aaptos is a genus of marine sponge which belongs to Suberitidae and is distributed in tropical and subtropical oceans. Bioactivity-guided fractionation of Aaptos sp. methanolic extract resulted in the isolation of aaptamine, demethyloxyaaptamine, and isoaaptamine. The cytotoxic activity of the isolated compounds was evaluated revealing that isoaaptamine exhibited potent cytotoxic activity against breast cancer T-47D cells. In a concentration-dependent manner, isoaaptamine inhibited the growth of T-47D cells as indicated by short-(MTT) and long-term (colony formation) anti-proliferative assays. The cytotoxic effect of isoaaptamine was mediated through apoptosis as indicated by DNA ladder formation, caspase-7 activation, XIAP inhibition and PARP cleavage. Transmission electron microscopy and flow cytometric analysis using acridine orange dye indicated that isoaaptamine treatment could induce T-47D cells autophagy. Immunoblot assays demonstrated that isoaaptamine treatment significantly activated autophagy marker proteins such as type II LC-3. In addition, isoaaptamine treatment enhanced the activation of DNA damage (γH2AX) and ER stress-related proteins (IRE1 α and BiP). Moreover, the use of isoaaptamine resulted in a significant increase in the generation of reactive oxygen species (ROS) as well as in the disruption of mitochondrial membrane potential (MMP). The pretreatment of T-47D cells with an ROS scavenger, N-acetyl-l-cysteine (NAC), attenuated the apoptosis and MMP disruption induced by isoaaptamine up to 90%, and these effects were mediated by the disruption of nuclear factor erythroid 2-related factor 2 (Nrf 2)/p62 pathway. Taken together, these findings suggested that the cytotoxic effect of isoaaptamine is associated with the induction of apoptosis and autophagy through oxidative stress. Our data indicated that isoaaptamine represents an interesting drug lead in the war against breast cancer.
Keywords: Nrf2/p62; ROS; anti-cancer; apoptosis; autophagy; isoaaptamine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
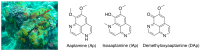





Similar articles
-
Antineoplastic agents. 380. Isolation and X-ray crystal structure determination of isoaaptamine from the Republic of Singapore Hymeniacidon sp. and conversion to the phosphate prodrug hystatin 1.J Nat Prod. 2004 Mar;67(3):506-9. doi: 10.1021/np0204592. J Nat Prod. 2004. PMID: 15043446
-
Juglanin induces apoptosis and autophagy in human breast cancer progression via ROS/JNK promotion.Biomed Pharmacother. 2017 Jan;85:303-312. doi: 10.1016/j.biopha.2016.11.030. Epub 2016 Nov 26. Biomed Pharmacother. 2017. PMID: 27899257
-
Towards the small and the beautiful: a small dibromotyrosine derivative from Pseudoceratina sp. sponge exhibits potent apoptotic effect through targeting IKK/NFκB signaling pathway.Mar Drugs. 2013 Aug 26;11(9):3168-85. doi: 10.3390/md11093168. Mar Drugs. 2013. PMID: 24065159 Free PMC article.
-
Oxidative stress-modulating drugs have preferential anticancer effects - involving the regulation of apoptosis, DNA damage, endoplasmic reticulum stress, autophagy, metabolism, and migration.Semin Cancer Biol. 2019 Oct;58:109-117. doi: 10.1016/j.semcancer.2018.08.010. Epub 2018 Aug 24. Semin Cancer Biol. 2019. PMID: 30149066 Review.
-
Recently confirmed apoptosis-inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment.Mar Drugs. 2011;9(9):1580-1606. doi: 10.3390/md9091580. Epub 2011 Sep 20. Mar Drugs. 2011. PMID: 22131960 Free PMC article. Review.
Cited by
-
New Drugs from the Sea: Pro-Apoptotic Activity of Sponges and Algae Derived Compounds.Mar Drugs. 2019 Jan 7;17(1):31. doi: 10.3390/md17010031. Mar Drugs. 2019. PMID: 30621025 Free PMC article. Review.
-
δ-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells.Cell Prolif. 2019 May;52(3):e12576. doi: 10.1111/cpr.12576. Epub 2019 Feb 4. Cell Prolif. 2019. PMID: 30719778 Free PMC article.
-
Withanolide C Inhibits Proliferation of Breast Cancer Cells via Oxidative Stress-Mediated Apoptosis and DNA Damage.Antioxidants (Basel). 2020 Sep 16;9(9):873. doi: 10.3390/antiox9090873. Antioxidants (Basel). 2020. PMID: 32947878 Free PMC article.
-
Methanol Extract of Clavularia inflata Exerts Apoptosis and DNA Damage to Oral Cancer Cells.Antioxidants (Basel). 2022 Sep 8;11(9):1777. doi: 10.3390/antiox11091777. Antioxidants (Basel). 2022. PMID: 36139851 Free PMC article.
-
Blue-Print Autophagy in 2020: A Critical Review.Mar Drugs. 2020 Sep 21;18(9):482. doi: 10.3390/md18090482. Mar Drugs. 2020. PMID: 32967369 Free PMC article. Review.
References
-
- Cheki M., Mihandoost E., Shirazi A., Mahmoudzadeh A. Prophylactic role of some plants and phytochemicals against radio-genotoxicity in human lymphocytes. J. Cancer Res. Ther. 2016;12:1234–1242. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

