Endogenous Reprogramming of Alpha Cells into Beta Cells, Induced by Viral Gene Therapy, Reverses Autoimmune Diabetes
- PMID: 29304344
- PMCID: PMC5757249
- DOI: 10.1016/j.stem.2017.11.020
Endogenous Reprogramming of Alpha Cells into Beta Cells, Induced by Viral Gene Therapy, Reverses Autoimmune Diabetes
Abstract
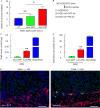
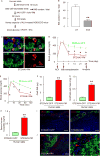
Successful strategies for treating type 1 diabetes need to restore the function of pancreatic beta cells that are destroyed by the immune system and overcome further destruction of insulin-producing cells. Here, we infused adeno-associated virus carrying Pdx1 and MafA expression cassettes through the pancreatic duct to reprogram alpha cells into functional beta cells and normalized blood glucose in both beta cell-toxin-induced diabetic mice and in autoimmune non-obese diabetic (NOD) mice. The euglycemia in toxin-induced diabetic mice and new insulin+ cells persisted in the autoimmune NOD mice for 4 months prior to reestablishment of autoimmune diabetes. This gene therapy strategy also induced alpha to beta cell conversion in toxin-treated human islets, which restored blood glucose levels in NOD/SCID mice upon transplantation. Hence, this strategy could represent a new therapeutic approach, perhaps complemented by immunosuppression, to bolster endogenous insulin production. Our study thus provides a potential basis for further investigation in human type 1 diabetes.
Keywords: MafA; NOD; Pdx1; adoptive transfer; alpha cells; beta cells; human islets; intraductal viral infusion; islet transplantation; lineage tracing.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Xiangwei Xiao, Ping Guo and George Gittes have a US national phase patent pending for intra-pancreatic ductal delivery of reagents through endoscopic retrograde cholangiopancreatography. PCT Application No. PCT/US2015/026532.
Figures
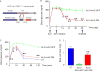
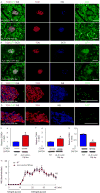
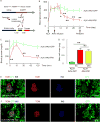

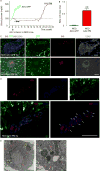
Comment in
-
Alpha to Beta Cell Reprogramming: Stepping toward a New Treatment for Diabetes.Cell Stem Cell. 2018 Jan 4;22(1):12-13. doi: 10.1016/j.stem.2017.12.012. Cell Stem Cell. 2018. PMID: 29304337
-
Gene therapy: Autoimmune diabetes reversed in mice.Nat Rev Endocrinol. 2018 Mar;14(3):127. doi: 10.1038/nrendo.2018.4. Epub 2018 Jan 19. Nat Rev Endocrinol. 2018. PMID: 29348473 No abstract available.
Similar articles
-
Glucagon blockade restores functional β-cell mass in type 1 diabetic mice and enhances function of human islets.Proc Natl Acad Sci U S A. 2021 Mar 2;118(9):e2022142118. doi: 10.1073/pnas.2022142118. Proc Natl Acad Sci U S A. 2021. PMID: 33619103 Free PMC article.
-
PDGF Facilitates Direct Lineage Reprogramming of Hepatocytes to Functional β-Like Cells Induced by Pdx1 and Ngn3.Cell Transplant. 2016 Oct;25(10):1893-1909. doi: 10.3727/096368916X691439. Cell Transplant. 2016. PMID: 27076404
-
Islet remodeling in female mice with spontaneous autoimmune and streptozotocin-induced diabetes.PLoS One. 2014 Aug 7;9(8):e102843. doi: 10.1371/journal.pone.0102843. eCollection 2014. PLoS One. 2014. PMID: 25101835 Free PMC article.
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
-
How to make insulin-producing pancreatic β cells for diabetes treatment.Sci China Life Sci. 2017 Mar;60(3):239-248. doi: 10.1007/s11427-016-0211-3. Epub 2016 Oct 27. Sci China Life Sci. 2017. PMID: 27796637 Review.
Cited by
-
The Powdered Root of Eurycoma longifolia Jack Improves Beta-Cell Number and Pancreatic Islet Performance through PDX1 Induction and Shows Antihyperglycemic Activity in db/db Mice.Nutrients. 2020 Jul 16;12(7):2111. doi: 10.3390/nu12072111. Nutrients. 2020. PMID: 32708678 Free PMC article.
-
Recent Advancements in mRNA Vaccines: From Target Selection to Delivery Systems.Vaccines (Basel). 2024 Aug 1;12(8):873. doi: 10.3390/vaccines12080873. Vaccines (Basel). 2024. PMID: 39203999 Free PMC article. Review.
-
Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors.JCI Insight. 2019 Nov 14;4(22):e131610. doi: 10.1172/jci.insight.131610. JCI Insight. 2019. PMID: 31723052 Free PMC article.
-
Rejuvenation of β cells by epigenetic editing.J Clin Invest. 2019 Jan 2;129(1):51-52. doi: 10.1172/JCI124583. Epub 2018 Nov 26. J Clin Invest. 2019. PMID: 30475229 Free PMC article.
-
How, When, and Where Do Human β-Cells Regenerate?Curr Diab Rep. 2019 Jun 27;19(8):48. doi: 10.1007/s11892-019-1176-8. Curr Diab Rep. 2019. PMID: 31250214 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

