Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss
- PMID: 29302038
- PMCID: PMC5754356
- DOI: 10.1038/s41467-017-02490-4
Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss
Abstract
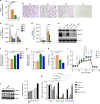
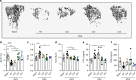
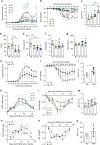
Microbial metabolites are known to modulate immune responses of the host. The main metabolites derived from microbial fermentation of dietary fibers in the intestine, short-chain fatty acids (SCFA), affect local and systemic immune functions. Here we show that SCFA are regulators of osteoclast metabolism and bone mass in vivo. Treatment of mice with SCFA as well as feeding with a high-fiber diet significantly increases bone mass and prevents postmenopausal and inflammation-induced bone loss. The protective effects of SCFA on bone mass are associated with inhibition of osteoclast differentiation and bone resorption in vitro and in vivo, while bone formation is not affected. Mechanistically, propionate (C3) and butyrate (C4) induce metabolic reprogramming of osteoclasts resulting in enhanced glycolysis at the expense of oxidative phosphorylation, thereby downregulating essential osteoclast genes such as TRAF6 and NFATc1. In summary, these data identify SCFA as potent regulators of osteoclast metabolism and bone homeostasis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Butyrate Inhibits Osteoclast Activity In Vitro and Regulates Systemic Inflammation and Bone Healing in a Murine Osteotomy Model Compared to Antibiotic-Treated Mice.Mediators Inflamm. 2021 Dec 10;2021:8817421. doi: 10.1155/2021/8817421. eCollection 2021. Mediators Inflamm. 2021. PMID: 34924815 Free PMC article.
-
Short-chain fatty acids and FFAR2 as suppressors of bone resorption.Bone. 2019 Aug;125:112-121. doi: 10.1016/j.bone.2019.05.016. Epub 2019 May 14. Bone. 2019. PMID: 31100533
-
RARγ is a negative regulator of osteoclastogenesis.J Steroid Biochem Mol Biol. 2015 Jun;150:46-53. doi: 10.1016/j.jsbmb.2015.03.005. Epub 2015 Mar 20. J Steroid Biochem Mol Biol. 2015. PMID: 25800721
-
Formation of propionate and butyrate by the human colonic microbiota.Environ Microbiol. 2017 Jan;19(1):29-41. doi: 10.1111/1462-2920.13589. Epub 2016 Dec 8. Environ Microbiol. 2017. PMID: 27928878 Review.
-
The role of short-chain fatty acids in the regulation of osteoporosis: new perspectives from gut microbiota to bone health: A review.Medicine (Baltimore). 2024 Aug 23;103(34):e39471. doi: 10.1097/MD.0000000000039471. Medicine (Baltimore). 2024. PMID: 39183408 Free PMC article. Review.
Cited by
-
Blackcurrant Anthocyanins Attenuate Estrogen -Deficiency-Induced Bone Loss through Modulating Microbial-Derived Short-Chain Carboxylic Acids and Phytoestrogen Metabolites in Peri- and Early Postmenopausal Women.Metabolites. 2024 Oct 11;14(10):541. doi: 10.3390/metabo14100541. Metabolites. 2024. PMID: 39452922 Free PMC article.
-
Anaerostipes caccae CML199 enhances bone development and counteracts aging-induced bone loss through the butyrate-driven gut-bone axis: the chicken model.Microbiome. 2024 Oct 22;12(1):215. doi: 10.1186/s40168-024-01920-y. Microbiome. 2024. PMID: 39438898 Free PMC article.
-
The role of short-chain fatty acid metabolism in the pathogenesis, diagnosis and treatment of cancer.Front Oncol. 2024 Oct 7;14:1451045. doi: 10.3389/fonc.2024.1451045. eCollection 2024. Front Oncol. 2024. PMID: 39435279 Free PMC article. Review.
-
Dihydromyricetin ameliorate postmenopausal osteoporosis in ovariectomized mice: Integrative microbiomic and metabolomic analysis.Front Pharmacol. 2024 Oct 2;15:1452921. doi: 10.3389/fphar.2024.1452921. eCollection 2024. Front Pharmacol. 2024. PMID: 39415843 Free PMC article.
-
Evaluation of Functional Components of Lactobacillus plantarum AR495 on Ovariectomy-Induced Osteoporosis in Mice And RAW264.7 Cells.Foods. 2024 Sep 29;13(19):3115. doi: 10.3390/foods13193115. Foods. 2024. PMID: 39410150 Free PMC article.
References
-
- Edwards CJ. Commensal gut bacteria and the etiopathogenesis of rheumatoid arthritis. J. Rheumatol. 2008;35:1477–14797. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

