A Role for Myosin Va in Human Cytomegalovirus Nuclear Egress
- PMID: 29298889
- PMCID: PMC5827399
- DOI: 10.1128/JVI.01849-17
A Role for Myosin Va in Human Cytomegalovirus Nuclear Egress
Abstract
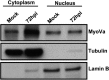
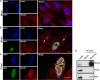
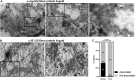
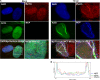
Herpesviruses replicate and package their genomes into capsids in replication compartments within the nuclear interior. Capsids then move to the inner nuclear membrane for envelopment and release into the cytoplasm in a process called nuclear egress. We previously found that nuclear F-actin is induced upon infection with the betaherpesvirus human cytomegalovirus (HCMV) and is important for nuclear egress and capsid localization away from replication compartment-like inclusions toward the nuclear rim. Despite these and related findings, it has not been shown that any specific motor protein is involved in herpesvirus nuclear egress. In this study, we have investigated whether the host motor protein, myosin Va, could be fulfilling this role. Using immunofluorescence microscopy and coimmunoprecipitation, we observed associations between a nuclear population of myosin Va and the viral major capsid protein, with both concentrating at the periphery of replication compartments. Immunoelectron microscopy showed that nearly 40% of assembled nuclear capsids associate with myosin Va. We also found that myosin Va and major capsid protein colocalize with nuclear F-actin. Importantly, antagonism of myosin Va with RNA interference or a dominant negative mutant revealed that myosin Va is important for the efficient production of infectious virus, capsid accumulation in the cytoplasm, and capsid localization away from replication compartment-like inclusions toward the nuclear rim. Our results lead us to suggest a working model whereby human cytomegalovirus capsids associate with myosin Va for movement from replication compartments to the nuclear periphery during nuclear egress.IMPORTANCE Little is known regarding how newly assembled and packaged herpesvirus capsids move from the nuclear interior to the periphery during nuclear egress. While it has been proposed that an actomyosin-based mechanism facilitates intranuclear movement of alphaherpesvirus capsids, a functional role for any specific myosin in nuclear egress has not been reported. Furthermore, the notion that an actomyosin-based mechanism facilitates intranuclear capsid movement is controversial. Here we show that human cytomegalovirus capsids associate with nuclear myosin Va and F-actin and that antagonism of myosin Va impairs capsid localization toward the nuclear rim and nuclear egress. Together with our previous results showing that nuclear F-actin is induced upon HCMV infection and is also important for these processes, our results lend support to the hypothesis that nascent human cytomegalovirus capsids migrate to the nuclear periphery via actomyosin-based movement. These results shed light on a poorly understood viral process and the cellular machinery involved.
Keywords: actin; cytomegalovirus; intranuclear movement; myosin; nuclear egress; replication compartment.
Copyright © 2018 American Society for Microbiology.
Figures



Similar articles
-
A Role for Nuclear F-Actin Induction in Human Cytomegalovirus Nuclear Egress.mBio. 2016 Aug 23;7(4):e01254-16. doi: 10.1128/mBio.01254-16. mBio. 2016. PMID: 27555312 Free PMC article.
-
Human Cytomegalovirus Nuclear Egress Complex Subunit, UL53, Associates with Capsids and Myosin Va, but Is Not Important for Capsid Localization towards the Nuclear Periphery.Viruses. 2022 Feb 26;14(3):479. doi: 10.3390/v14030479. Viruses. 2022. PMID: 35336886 Free PMC article.
-
Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97.Viruses. 2018 Jan 13;10(1):35. doi: 10.3390/v10010035. Viruses. 2018. PMID: 29342872 Free PMC article.
-
Getting to and through the inner nuclear membrane during herpesvirus nuclear egress.Curr Opin Cell Biol. 2017 Jun;46:9-16. doi: 10.1016/j.ceb.2016.12.007. Epub 2017 Jan 10. Curr Opin Cell Biol. 2017. PMID: 28086162 Free PMC article. Review.
-
The human cytomegalovirus nuclear egress complex unites multiple functions: Recruitment of effectors, nuclear envelope rearrangement, and docking to nuclear capsids.Rev Med Virol. 2017 Jul;27(4). doi: 10.1002/rmv.1934. Epub 2017 Jun 30. Rev Med Virol. 2017. PMID: 28664574 Review.
Cited by
-
Human Cytomegalovirus Egress: Overcoming Barriers and Co-Opting Cellular Functions.Viruses. 2021 Dec 22;14(1):15. doi: 10.3390/v14010015. Viruses. 2021. PMID: 35062219 Free PMC article. Review.
-
Rho-Associated Coiled-Coil Kinase 1 Translocates to the Nucleus and Inhibits Human Cytomegalovirus Propagation.J Virol. 2019 Sep 12;93(19):e00453-19. doi: 10.1128/JVI.00453-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31292242 Free PMC article.
-
A putative WAVE regulatory complex (WRC) interacting receptor sequence (WIRS) in the cytoplasmic tail of HSV-1 gE does not function in WRC recruitment or neuronal transport.Access Microbiol. 2021 Mar 4;3(3):000206. doi: 10.1099/acmi.0.000206. eCollection 2021 Mar. Access Microbiol. 2021. PMID: 34151161 Free PMC article.
-
Virus-host protein interactions as footprints of human cytomegalovirus replication.Curr Opin Virol. 2022 Feb;52:135-147. doi: 10.1016/j.coviro.2021.11.016. Epub 2021 Dec 16. Curr Opin Virol. 2022. PMID: 34923282 Free PMC article. Review.
-
Emerging roles of cytoskeletal transport and scaffold systems in human viral propagation.Anim Cells Syst (Seoul). 2024 Oct 21;28(1):506-518. doi: 10.1080/19768354.2024.2418332. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 39439927 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

