Circulating tumor cells: potential markers of minimal residual disease in ovarian cancer? a study of the OVCAD consortium
- PMID: 29290959
- PMCID: PMC5739744
- DOI: 10.18632/oncotarget.22468
Circulating tumor cells: potential markers of minimal residual disease in ovarian cancer? a study of the OVCAD consortium
Abstract
Purpose: In 75% of ovarian cancer patients the tumor mass is completely eradicated by established surgical and cytotoxic treatment; however, the majority of the tumors recur within 24 months. Here we investigated the role of circulating tumor cells (CTCs) indicating occult tumor load, which remains inaccessible by established diagnostics.
Experimental design: Blood was taken at diagnosis (baseline samples, n = 102) and six months after completion of adjuvant first-line chemotherapy (follow-up samples; n = 78). CTCs were enriched by density gradient centrifugation. A multi-marker immunostaining was established and further complemented by FISH on CTCs and tumor/metastasis tissues using probes for stem-cell like fusion genes MECOM and HHLA1.
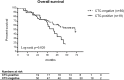
Results: CTCs were observed in 26.5% baseline and 7.7% follow-up blood samples at a mean number of 12.4 and 2.8 CTCs per ml blood, respectively. Baseline CTCs indicated a higher risk of death in R0 patients with complete gross resection (univariate: HR 2.158, 95% CI 1.111-4.191, p = 0.023; multivariate: HR 2.720, 95% CI 1.340-5.522, p = 0.006). At follow-up, the presence of CTCs was associated with response to primary treatment as assessed using RECIST criteria. Chromosomal gains at MECOM and HHLA1 loci suggest that the observed cells were cancer cells and reflect pathophysiological decisive chromosomal aberrations of the primary and metastatic tumors.
Conclusions: Our data suggest that CTCs detected by the multi-marker protein panel and/or MECOM/HHLA1 FISH represent minimal residual disease in optimally debulked ovarian cancer patients. The role of CTCs cells especially for clinical therapy stratification of the patients has to be validated in consecutive larger studies applying standardized treatment schemes.
Keywords: FISH on CTCs; circulating tumour cells; minimal residual disease; multi-marker analysis; ovarian cancer.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no potential conflicts of interest.
Figures

Similar articles
-
Gene expression profiling of single circulating tumor cells in ovarian cancer - Establishment of a multi-marker gene panel.Mol Oncol. 2016 Aug;10(7):1030-42. doi: 10.1016/j.molonc.2016.04.002. Epub 2016 Apr 20. Mol Oncol. 2016. PMID: 27157930 Free PMC article.
-
The Long-Term Prognostic Significance of Circulating Tumor Cells in Ovarian Cancer-A Study of the OVCAD Consortium.Cancers (Basel). 2021 May 26;13(11):2613. doi: 10.3390/cancers13112613. Cancers (Basel). 2021. PMID: 34073412 Free PMC article.
-
Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance -- a study of the OVCAD consortium.Gynecol Oncol. 2013 Jan;128(1):15-21. doi: 10.1016/j.ygyno.2012.09.021. Epub 2012 Sep 24. Gynecol Oncol. 2013. PMID: 23017820
-
Circulating and disseminated tumor cells in ovarian cancer: a systematic review.Gynecol Oncol. 2014 Jun;133(3):632-9. doi: 10.1016/j.ygyno.2014.03.016. Epub 2014 Mar 19. Gynecol Oncol. 2014. PMID: 24657303 Review.
-
Circulating tumour cells in breast cancer--review.Klin Onkol. 2010;23(2):86-91. Klin Onkol. 2010. PMID: 20465086 Review.
Cited by
-
Potential clinical utility of liquid biopsies in ovarian cancer.Mol Cancer. 2022 May 11;21(1):114. doi: 10.1186/s12943-022-01588-8. Mol Cancer. 2022. PMID: 35545786 Free PMC article. Review.
-
Establishment of an optimized CTC detection model consisting of EpCAM, MUC1 and WT1 in epithelial ovarian cancer and its correlation with clinical characteristics.Chin J Cancer Res. 2022 Apr 30;34(2):95-108. doi: 10.21147/j.issn.1000-9604.2022.02.04. Chin J Cancer Res. 2022. PMID: 35685992 Free PMC article.
-
Advances and challenges in the use of liquid biopsy in gynaecological oncology.Heliyon. 2024 Oct 15;10(20):e39148. doi: 10.1016/j.heliyon.2024.e39148. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39492906 Free PMC article. Review.
-
Circulating tumor cells and cell-free nucleic acids in patients with gynecological malignancies.Virchows Arch. 2018 Oct;473(4):395-403. doi: 10.1007/s00428-018-2447-5. Epub 2018 Aug 25. Virchows Arch. 2018. PMID: 30145616 Review.
-
High-Grade Serous Ovarian Cancer-A Risk Factor Puzzle and Screening Fugitive.Biomedicines. 2024 Jan 19;12(1):229. doi: 10.3390/biomedicines12010229. Biomedicines. 2024. PMID: 38275400 Free PMC article. Review.
References
-
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. https://doi.org/10.1016/j.ejca.2009.12.014. - DOI - PubMed
-
- Vergote I, Van Gorp T, Cadron I, Leunen K, Neven P, Amant F. Improving outcome in the first-line management of advanced ovarian cancer. Eur J Cancer. 2007;5:23–8. https://doi.org/10.1016/s1359-6349(07)70012-5. - DOI
-
- Polterauer S, Vergote I, Concin N, Braicu I, Chekerov R, Mahner S, Woelber L, Cadron I, Van Gorp T, Zeillinger R, Castillo-Tong DC, Sehouli J. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int J Gynecol Cancer. 2012;22:380–5. https://doi.org/10.1097/IGC.0b013e31823de6ae. - DOI - PubMed
-
- Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, Kristensen G, Mediola C, Coens C, Qian W, Parmar MK, Swart AM. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376:1155–63. https://doi.org/10.1016/S0140-6736(10)61268-8. - DOI - PubMed
-
- Paterlini-Brechot P, Benali N. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253:180–204. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

