Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function
- PMID: 29290800
- PMCID: PMC5743467
- DOI: 10.7150/thno.21234
Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function
Abstract
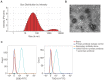
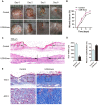
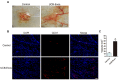
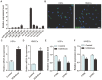
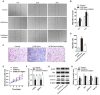
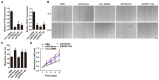
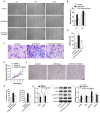
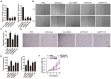
The application of blood plasma for soft tissue wound healing is receiving much more attention recently. Exosomes are critical paracrine mediators that can be obtained from biological fluids including plasma and be able to induce regenerative effects by transferring bioactive molecules such as microRNAs (miRNAs). This study aimed to investigate the effects of exosomes from human umbilical cord blood plasma (UCB-Exos) on wound healing and to elucidate the underlying mechanism. Methods: UCB-Exos were isolated by ultracentrifugation and subcutaneously injected into full-thickness skin wounds in mice. The efficacy of UCB-Exos on wound healing was evaluated by measuring wound closure rates, histological analysis and immunofluorescence examinations. In vitro, quantitative real-time PCR (qRT-PCR) analysis was performed to detect the expression levels of a class of miRNAs that have positive roles in regulating wound healing. The scratch wound assay, transwell assay and cell counting kit-8 analysis were conducted to assess the effects of UCB-Exos on migration and proliferation of human skin fibroblasts and endothelial cells. Tube formation assay was carried out to test the impact of UCB-Exos on angiogenic tube formation ability of endothelial cells. Meanwhile, by using specific RNA inhibitors or siRNAs, the roles of the candidate miRNA and its target genes in UCB-Exos-induced regulation of function of fibroblasts and endothelial cells were assessed. Results: The local transplantation of UCB-Exos into mouse skin wounds resulted in accelerated re-epithelialization, reduced scar widths, and enhanced angiogenesis. In vitro, UCB-Exos could promote the proliferation and migration of fibroblasts, and enhance the angiogenic activities of endothelial cells. Notably, miR-21-3p was found to be highly enriched in UCB-Exos and served as a critical mediator in UCB-Exos -induced regulatory effects through inhibition of phosphatase and tensin homolog (PTEN) and sprouty homolog 1 (SPRY1). Conclusion: Our results suggest that UCB-Exos are important effectors of plasma activity and can be used as a novel promising strategy for soft tissue wound healing.
Keywords: cord blood; exosomes; miR-21-3p.; wound healing.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis.J Transl Med. 2015 Feb 1;13:49. doi: 10.1186/s12967-015-0417-0. J Transl Med. 2015. PMID: 25638205 Free PMC article.
-
Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation.Theranostics. 2019 Sep 21;9(23):6901-6919. doi: 10.7150/thno.37357. eCollection 2019. Theranostics. 2019. PMID: 31660076 Free PMC article.
-
Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis.Theranostics. 2018 Feb 7;8(6):1607-1623. doi: 10.7150/thno.22958. eCollection 2018. Theranostics. 2018. PMID: 29556344 Free PMC article.
-
Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review.J Cosmet Dermatol. 2020 Mar;19(3):574-581. doi: 10.1111/jocd.13215. Epub 2019 Nov 21. J Cosmet Dermatol. 2020. PMID: 31755172 Review.
-
Exosomes from adipose-derived stem cells and application to skin wound healing.Cell Prolif. 2021 Mar;54(3):e12993. doi: 10.1111/cpr.12993. Epub 2021 Jan 17. Cell Prolif. 2021. PMID: 33458899 Free PMC article. Review.
Cited by
-
Exosomes derived from dental pulp stem cells accelerate cutaneous wound healing by enhancing angiogenesis via the Cdc42/p38 MAPK pathway.Int J Mol Med. 2022 Dec;50(6):143. doi: 10.3892/ijmm.2022.5199. Epub 2022 Nov 2. Int J Mol Med. 2022. PMID: 36321793 Free PMC article.
-
VPS4B mutation impairs the osteogenic differentiation of dental follicle cells derived from a patient with dentin dysplasia type I.Int J Oral Sci. 2020 Jul 31;12(1):22. doi: 10.1038/s41368-020-00088-z. Int J Oral Sci. 2020. PMID: 32737282 Free PMC article.
-
MSC-Derived Extracellular Vesicles to Heal Diabetic Wounds: a Systematic Review and Meta-Analysis of Preclinical Animal Studies.Stem Cell Rev Rep. 2022 Mar;18(3):968-979. doi: 10.1007/s12015-021-10164-4. Epub 2021 Apr 24. Stem Cell Rev Rep. 2022. PMID: 33893619 Free PMC article.
-
N6‑methyladenosine‑induced long non‑coding RNA PVT1 regulates the miR‑27b‑3p/BLM axis to promote prostate cancer progression.Int J Oncol. 2023 Jan;62(1):16. doi: 10.3892/ijo.2022.5464. Epub 2022 Dec 9. Int J Oncol. 2023. PMID: 36484368 Free PMC article.
-
Aerobic exercise-induced circulating extracellular vesicle combined decellularized dermal matrix hydrogel facilitates diabetic wound healing by promoting angiogenesis.Front Bioeng Biotechnol. 2022 Aug 23;10:903779. doi: 10.3389/fbioe.2022.903779. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36082169 Free PMC article.
References
-
- van Zanten MC, Mistry RM, Suami H, Campbell-Lloyd A, Finkemeyer JP, Piller NB. et al. The Lymphatic Response to Injury with Soft-Tissue Reconstruction in High-Energy Open Tibial Fractures of the Lower Extremity. Plast Reconstr Surg. 2017;139:483–91. - PubMed
-
- Bhandari M, Petrisor BA, Jeray KJ. Wound Irrigation in Initial Management of Open Fractures. N Engl J Med. 2016;374:1789–90. - PubMed
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

