Host triacylglycerols shape the lipidome of intracellular trypanosomes and modulate their growth
- PMID: 29281741
- PMCID: PMC5760102
- DOI: 10.1371/journal.ppat.1006800
Host triacylglycerols shape the lipidome of intracellular trypanosomes and modulate their growth
Abstract
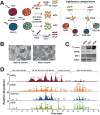
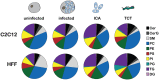
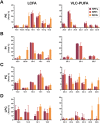
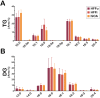
Intracellular infection and multi-organ colonization by the protozoan parasite, Trypanosoma cruzi, underlie the complex etiology of human Chagas disease. While T. cruzi can establish cytosolic residence in a broad range of mammalian cell types, the molecular mechanisms governing this process remain poorly understood. Despite the anticipated capacity for fatty acid synthesis in this parasite, recent observations suggest that intracellular T. cruzi amastigotes may rely on host fatty acid metabolism to support infection. To investigate this prediction, it was necessary to establish baseline lipidome information for the mammalian-infective stages of T. cruzi and their mammalian host cells. An unbiased, quantitative mass spectrometric analysis of lipid fractions was performed with the identification of 1079 lipids within 30 classes. From these profiles we deduced that T. cruzi amastigotes maintain an overall lipid identity that is distinguishable from mammalian host cells. A deeper analysis of the fatty acid moiety distributions within each lipid subclass facilitated the high confidence assignment of host- and parasite-like lipid signatures. This analysis unexpectedly revealed a strong host lipid signature in the parasite lipidome, most notably within its glycerolipid fraction. The near complete overlap of fatty acid moiety distributions observed for host and parasite triacylglycerols suggested that T. cruzi amastigotes acquired a significant portion of their lipidome from host triacylglycerol pools. Metabolic tracer studies confirmed long-chain fatty acid scavenging by intracellular T. cruzi amastigotes, a capacity that was significantly diminished in host cells deficient for de novo triacylglycerol synthesis via the diacylglycerol acyltransferases (DGAT1/2). Reduced T. cruzi amastigote proliferation in DGAT1/2-deficient fibroblasts further underscored the importance of parasite coupling to host triacylglycerol pools during the intracellular infection cycle. Thus, our comprehensive lipidomic dataset provides a substantially enhanced view of T. cruzi infection biology highlighting the interplay between host and parasite lipid metabolism with potential bearing on future therapeutic intervention strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Modulation of host central carbon metabolism and in situ glucose uptake by intracellular Trypanosoma cruzi amastigotes.PLoS Pathog. 2017 Nov 27;13(11):e1006747. doi: 10.1371/journal.ppat.1006747. eCollection 2017 Nov. PLoS Pathog. 2017. PMID: 29176805 Free PMC article.
-
Transcriptome Remodeling in Trypanosoma cruzi and Human Cells during Intracellular Infection.PLoS Pathog. 2016 Apr 5;12(4):e1005511. doi: 10.1371/journal.ppat.1005511. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27046031 Free PMC article.
-
Targeting host mitochondria: A role for the Trypanosoma cruzi amastigote flagellum.Cell Microbiol. 2018 Feb;20(2):10.1111/cmi.12807. doi: 10.1111/cmi.12807. Epub 2017 Nov 28. Cell Microbiol. 2018. PMID: 29119655 Free PMC article.
-
Autophagy: A necessary process during the Trypanosoma cruzi life-cycle.Virulence. 2019 Dec;10(1):460-469. doi: 10.1080/21505594.2018.1543517. Epub 2018 Nov 29. Virulence. 2019. PMID: 30489206 Free PMC article. Review.
-
Mechanisms of host cell invasion by Trypanosoma cruzi.Adv Parasitol. 2011;76:33-61. doi: 10.1016/B978-0-12-385895-5.00002-5. Adv Parasitol. 2011. PMID: 21884886 Review.
Cited by
-
Comparative transcriptomics and host-specific parasite gene expression profiles inform on drivers of proliferative kidney disease.Sci Rep. 2021 Jan 25;11(1):2149. doi: 10.1038/s41598-020-77881-7. Sci Rep. 2021. PMID: 33495500 Free PMC article.
-
De Novo Synthesis of Phosphatidylcholine Is Essential for the Promastigote But Not Amastigote Stage in Leishmania major.Front Cell Infect Microbiol. 2021 Mar 12;11:647870. doi: 10.3389/fcimb.2021.647870. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33777852 Free PMC article.
-
Lipidomic changes in the liver of beagle dogs associated with Toxocara canis infection.Front Cell Infect Microbiol. 2022 Sep 13;12:890589. doi: 10.3389/fcimb.2022.890589. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36176575 Free PMC article.
-
Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids.Membranes (Basel). 2021 Nov 24;11(12):919. doi: 10.3390/membranes11120919. Membranes (Basel). 2021. PMID: 34940418 Free PMC article. Review.
-
Molecular dissection of Chagas induced cardiomyopathy reveals central disease associated and druggable signaling pathways.PLoS Negl Trop Dis. 2020 May 20;14(5):e0007980. doi: 10.1371/journal.pntd.0007980. eCollection 2020 May. PLoS Negl Trop Dis. 2020. PMID: 32433643 Free PMC article.
References
-
- Nunes MC, Guimaraes MH Junior, Diamantino AC, Gelape CL, Ferrari TC. Cardiac manifestations of parasitic diseases. Heart. 2017; 103(9):651–8. doi: 10.1136/heartjnl-2016-309870 . - DOI - PubMed
-
- World Health Organization WHO. Chagas Disease (American Trypanosomiasis) 2017 [29 Mar 2017]. http://www.who.int/mediacentre/factsheets/fs340/en/.
-
- Pecoul B, Batista C, Stobbaerts E, Ribeiro I, Vilasanjuan R, Gascon J, et al. The BENEFIT Trial: Where Do We Go from Here? PLoS Negl Trop Dis. 2016;10(2):e0004343 doi: 10.1371/journal.pntd.0004343 . - DOI - PMC - PubMed
-
- Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. 2013;13(4):342–8. doi: 10.1016/S1473-3099(13)70002-1 . - DOI - PMC - PubMed
-
- Prata A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect Dis. 2001;1(2):92–100. doi: 10.1016/S1473-3099(01)00065-2 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

