A mouse model of DEPDC5-related epilepsy: Neuronal loss of Depdc5 causes dysplastic and ectopic neurons, increased mTOR signaling, and seizure susceptibility
- PMID: 29274432
- PMCID: PMC5803417
- DOI: 10.1016/j.nbd.2017.12.010
A mouse model of DEPDC5-related epilepsy: Neuronal loss of Depdc5 causes dysplastic and ectopic neurons, increased mTOR signaling, and seizure susceptibility
Abstract
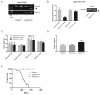
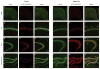
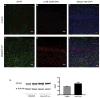
DEPDC5 is a newly identified epilepsy-related gene implicated in focal epilepsy, brain malformations, and Sudden Unexplained Death in Epilepsy (SUDEP). In vitro, DEPDC5 negatively regulates amino acid sensing by the mTOR complex 1 (mTORC1) pathway, but the role of DEPDC5 in neurodevelopment and epilepsy has not been described. No animal model of DEPDC5-related epilepsy has recapitulated the neurological phenotypes seen in patients, and germline knockout rodent models are embryonic lethal. Here, we establish a neuron-specific Depdc5 conditional knockout mouse by cre-recombination under the Synapsin1 promotor. Depdc5flox/flox-Syn1Cre (Depdc5cc+) mice survive to adulthood with a progressive neurologic phenotype that includes motor abnormalities (i.e., hind limb clasping) and reduced survival compared to littermate control mice. Depdc5cc+ mice have larger brains with increased cortical neuron size and dysplastic neurons throughout the cortex, comparable to the abnormal neurons seen in human focal cortical dysplasia specimens. Depdc5 results in constitutive mTORC1 hyperactivation exclusively in neurons as measured by the increased phosphorylation of the downstream ribosomal protein S6. Despite a lack of increased mTORC1 signaling within astrocytes, Depdc5cc+ brains show reactive astrogliosis. We observed two Depdc5cc+ mice to have spontaneous seizures, including a terminal seizure. We demonstrate that as a group Depdc5cc+ mice have lowered seizure thresholds, as evidenced by decreased latency to seizures after chemoconvulsant injection and increased mortality from pentylenetetrazole-induced seizures. In summary, our neuron-specific Depdc5 knockout mouse model recapitulates clinical, pathological, and biochemical features of human DEPDC5-related epilepsy and brain malformations. We thereby present an important model in which to study targeted therapeutic strategies for DEPDC5-related conditions.
Keywords: Conditional knockout; DEPDC5; Familial focal epilepsy; Focal cortical dysplasia; Megalencephaly; Seizures; mTOR.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Delving Deeper into DEPDC5.Epilepsy Curr. 2018 May-Jun;18(3):197-199. doi: 10.5698/1535-7597.18.3.197. Epilepsy Curr. 2018. PMID: 29950950 Free PMC article. No abstract available.
Similar articles
-
Chronic mTORC1 inhibition rescues behavioral and biochemical deficits resulting from neuronal Depdc5 loss in mice.Hum Mol Genet. 2019 Sep 1;28(17):2952-2964. doi: 10.1093/hmg/ddz123. Hum Mol Genet. 2019. PMID: 31174205 Free PMC article.
-
Prevention of premature death and seizures in a Depdc5 mouse epilepsy model through inhibition of mTORC1.Hum Mol Genet. 2020 May 28;29(8):1365-1377. doi: 10.1093/hmg/ddaa068. Hum Mol Genet. 2020. PMID: 32280987 Free PMC article.
-
Second-hit mosaic mutation in mTORC1 repressor DEPDC5 causes focal cortical dysplasia-associated epilepsy.J Clin Invest. 2018 Jun 1;128(6):2452-2458. doi: 10.1172/JCI99384. Epub 2018 Apr 30. J Clin Invest. 2018. PMID: 29708508 Free PMC article. Clinical Trial.
-
GATORopathies: The role of amino acid regulatory gene mutations in epilepsy and cortical malformations.Epilepsia. 2019 Nov;60(11):2163-2173. doi: 10.1111/epi.16370. Epub 2019 Oct 17. Epilepsia. 2019. PMID: 31625153 Free PMC article. Review.
-
mTOR signaling pathway genes in focal epilepsies.Prog Brain Res. 2016;226:61-79. doi: 10.1016/bs.pbr.2016.04.013. Epub 2016 Jun 7. Prog Brain Res. 2016. PMID: 27323939 Review.
Cited by
-
The mTOR pathway genes MTOR, Rheb, Depdc5, Pten, and Tsc1 have convergent and divergent impacts on cortical neuron development and function.Elife. 2024 Feb 27;12:RP91010. doi: 10.7554/eLife.91010. Elife. 2024. PMID: 38411613 Free PMC article.
-
[Role and mechanism of histone deacetylases in mouse neuronal development].Zhongguo Dang Dai Er Ke Za Zhi. 2021 Mar;23(3):294-299. doi: 10.7499/j.issn.1008-8830.2011098. Zhongguo Dang Dai Er Ke Za Zhi. 2021. PMID: 33691925 Free PMC article. Chinese.
-
NPRL3 loss alters neuronal morphology, mTOR localization, cortical lamination and seizure threshold.Brain. 2022 Nov 21;145(11):3872-3885. doi: 10.1093/brain/awac044. Brain. 2022. PMID: 35136953 Free PMC article.
-
Regulation of lifespan by neural excitation and REST.Nature. 2019 Oct;574(7778):359-364. doi: 10.1038/s41586-019-1647-8. Epub 2019 Oct 16. Nature. 2019. PMID: 31619788 Free PMC article.
-
Modeling Epilepsy Using Human Induced Pluripotent Stem Cells-Derived Neuronal Cultures Carrying Mutations in Ion Channels and the Mechanistic Target of Rapamycin Pathway.Front Mol Neurosci. 2022 Mar 10;15:810081. doi: 10.3389/fnmol.2022.810081. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35359577 Free PMC article. Review.
References
-
- Amin S, et al. Causes of mortality in individuals with tuberous sclerosis complex. Dev Med Child Neurol. 2017;59:612–617. - PubMed
-
- Bagnall RD, et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol. 2016;79:522–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

