Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex
- PMID: 29269727
- PMCID: PMC5740170
- DOI: 10.1038/s41467-017-02325-2
Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex
Abstract
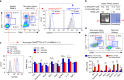
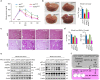
Reactive oxygen species (ROS) contribute to the development of non-alcoholic fatty liver disease. ROS generation by infiltrating macrophages involves multiple mechanisms, including Toll-like receptor 4 (TLR4)-mediated NADPH oxidase (NOX) activation. Here, we show that palmitate-stimulated CD11b+F4/80low hepatic infiltrating macrophages, but not CD11b+F4/80high Kupffer cells, generate ROS via dynamin-mediated endocytosis of TLR4 and NOX2, independently from MyD88 and TRIF. We demonstrate that differently from LPS-mediated dimerization of the TLR4-MD2 complex, palmitate binds a monomeric TLR4-MD2 complex that triggers endocytosis, ROS generation and increases pro-interleukin-1β expression in macrophages. Palmitate-induced ROS generation in human CD68lowCD14high macrophages is strongly suppressed by inhibition of dynamin. Furthermore, Nox2-deficient mice are protected against high-fat diet-induced hepatic steatosis and insulin resistance. Therefore, endocytosis of TLR4 and NOX2 into macrophages might be a novel therapeutic target for non-alcoholic fatty liver disease.
Conflict of interest statement
The authors declare no competing financial interests
Figures


Similar articles
-
Gliptins Suppress Inflammatory Macrophage Activation to Mitigate Inflammation, Fibrosis, Oxidative Stress, and Vascular Dysfunction in Models of Nonalcoholic Steatohepatitis and Liver Fibrosis.Antioxid Redox Signal. 2018 Jan 10;28(2):87-109. doi: 10.1089/ars.2016.6953. Epub 2017 Jul 25. Antioxid Redox Signal. 2018. PMID: 28635324
-
Arachidonic acid inhibits inflammatory responses by binding to myeloid differentiation factor-2 (MD2) and preventing MD2/toll-like receptor 4 signaling activation.Biochim Biophys Acta Mol Basis Dis. 2020 May 1;1866(5):165683. doi: 10.1016/j.bbadis.2020.165683. Epub 2020 Jan 14. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 31953218
-
Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2.Circ Res. 2009 Jan 30;104(2):210-8, 21p following 218. doi: 10.1161/CIRCRESAHA.108.181040. Epub 2008 Dec 18. Circ Res. 2009. PMID: 19096031 Free PMC article.
-
Monoclonal Antibody to CD14, TLR4, or CD11b: Impact of Epitope and Isotype Specificity on ROS Generation by Human Granulocytes and Monocytes.Oxid Med Cell Longev. 2020 Nov 20;2020:5708692. doi: 10.1155/2020/5708692. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33294123 Free PMC article. Review.
-
Myeloid differentiation factor 2 in the heart: Bench to bedside evidence for potential clinical benefits?Pharmacol Res. 2021 Jan;163:105239. doi: 10.1016/j.phrs.2020.105239. Epub 2020 Oct 11. Pharmacol Res. 2021. PMID: 33053443 Review.
Cited by
-
High levels of serum hypersensitive C-reactive protein are associated with non-alcoholic fatty liver disease in non-obese people: a cross-sectional study.Eur J Med Res. 2024 Oct 15;29(1):496. doi: 10.1186/s40001-024-02065-2. Eur J Med Res. 2024. PMID: 39402650 Free PMC article.
-
Experimental Applications of in situ Liver Perfusion Machinery for the Study of Liver Disease.Mol Cells. 2019 Jan 31;42(1):45-55. doi: 10.14348/molcells.2018.0330. Epub 2018 Dec 12. Mol Cells. 2019. PMID: 30665288 Free PMC article.
-
Molecular Mechanism Pathways of Natural Compounds for the Treatment of Non-Alcoholic Fatty Liver Disease.Molecules. 2023 Jul 25;28(15):5645. doi: 10.3390/molecules28155645. Molecules. 2023. PMID: 37570615 Free PMC article. Review.
-
Downregulation of 15-PGDH enhances MASH-HCC development via fatty acid-induced T-cell exhaustion.JHEP Rep. 2023 Aug 23;5(12):100892. doi: 10.1016/j.jhepr.2023.100892. eCollection 2023 Dec. JHEP Rep. 2023. PMID: 37942226 Free PMC article.
-
Myeloid cell MHC I expression drives CD8+ T cell activation in nonalcoholic steatohepatitis.Front Immunol. 2024 Jan 11;14:1302006. doi: 10.3389/fimmu.2023.1302006. eCollection 2023. Front Immunol. 2024. PMID: 38274832 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

