Clinical and Biological Manifestation of RNF168 Deficiency in Two Polish Siblings
- PMID: 29255463
- PMCID: PMC5722808
- DOI: 10.3389/fimmu.2017.01683
Clinical and Biological Manifestation of RNF168 Deficiency in Two Polish Siblings
Abstract
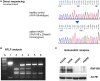
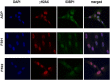
Germline mutations in the RING finger protein gene RNF168 have been identified in a combined immunodeficiency disorder called RIDDLE syndrome. Since only two patients have been described with somewhat different phenotypes, there is need to identify further patients. Here, we report on two Polish siblings with RNF168 deficiency due to homozygosity for a novel frameshift mutation, c.295delG, that was identified through exome sequencing. Both patients presented with immunoglobulin deficiency, telangiectasia, cellular radiosensitivity, and increased alpha-fetoprotein (AFP) levels. The younger sibling had a more pronounced neurological and morphological phenotype, and she also carried an ATM gene mutation in the heterozygous state. Immunoblot analyses showed absence of RNF168 protein, whereas ATM levels and function were proficient in lymphoblastoid cells from both patients. Consistent with the absence of RNF168 protein, 53BP1 recruitment to DNA double-strand breaks (DSBs) after irradiation was undetectable in lymphoblasts or primary fibroblasts from either of the two patients. γH2AX foci accumulated normally but they disappeared with significant delay, indicating a severe defect in DSB repair. A comparison with the two previously identified patients indicates immunoglobulin deficiency, cellular radiosensitivity, and increased AFP levels as hallmarks of RNF168 deficiency. The variability in its clinical expression despite similar cellular phenotypes suggests that some manifestations of RNF168 deficiency may be modified by additional genetic or epidemiological factors.
Keywords: DNA repair; chromosome instability; double-strand break repair; immunodeficiency syndrome; radiosensitivity.
Figures

Similar articles
-
Homozygous deficiency of ubiquitin-ligase ring-finger protein RNF168 mimics the radiosensitivity syndrome of ataxia-telangiectasia.Cell Death Differ. 2011 Sep;18(9):1500-6. doi: 10.1038/cdd.2011.18. Epub 2011 Mar 11. Cell Death Differ. 2011. PMID: 21394101 Free PMC article.
-
The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage.Cell. 2009 Feb 6;136(3):420-34. doi: 10.1016/j.cell.2008.12.042. Cell. 2009. PMID: 19203578
-
DNA double-strand break repair, immunodeficiency and the RIDDLE syndrome.Expert Rev Clin Immunol. 2011 Mar;7(2):169-85. doi: 10.1586/eci.10.93. Expert Rev Clin Immunol. 2011. PMID: 21426255 Review.
-
Opposing roles of RNF8/RNF168 and deubiquitinating enzymes in ubiquitination-dependent DNA double-strand break response signaling and DNA-repair pathway choice.J Radiat Res. 2016 Aug;57 Suppl 1(Suppl 1):i33-i40. doi: 10.1093/jrr/rrw027. Epub 2016 Mar 16. J Radiat Res. 2016. PMID: 26983989 Free PMC article. Review.
-
DNA double-strand break induction and repair in irradiated lymphoblastoid, fibroblast cell lines and white blood cells from ATM, NBS and radiosensitive patients.Strahlenther Onkol. 2007 Aug;183(8):447-53. doi: 10.1007/s00066-007-1683-4. Strahlenther Onkol. 2007. PMID: 17680225
Cited by
-
The ubiquitin codes in cellular stress responses.Protein Cell. 2024 Feb 29;15(3):157-190. doi: 10.1093/procel/pwad045. Protein Cell. 2024. PMID: 37470788 Free PMC article. Review.
-
New answers to the old RIDDLE: RNF168 and the DNA damage response pathway.FEBS J. 2022 May;289(9):2467-2480. doi: 10.1111/febs.15857. Epub 2021 Apr 16. FEBS J. 2022. PMID: 33797206 Free PMC article. Review.
-
Defective repair of topoisomerase I induced chromosomal damage in Huntington's disease.Cell Mol Life Sci. 2022 Feb 28;79(3):160. doi: 10.1007/s00018-022-04204-6. Cell Mol Life Sci. 2022. PMID: 35224690 Free PMC article.
-
Moving the Needle Forward in Genomically-Guided Precision Radiation Treatment.Cancers (Basel). 2023 Nov 7;15(22):5314. doi: 10.3390/cancers15225314. Cancers (Basel). 2023. PMID: 38001574 Free PMC article. Review.
-
Human RAD50 deficiency: Confirmation of a distinctive phenotype.Am J Med Genet A. 2020 Jun;182(6):1378-1386. doi: 10.1002/ajmg.a.61570. Epub 2020 Mar 25. Am J Med Genet A. 2020. PMID: 32212377 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

