X-linked inhibitor of apoptosis protein (XIAP) is a client of heat shock protein 70 (Hsp70) and a biomarker of its inhibition
- PMID: 29255093
- PMCID: PMC5818200
- DOI: 10.1074/jbc.RA117.000634
X-linked inhibitor of apoptosis protein (XIAP) is a client of heat shock protein 70 (Hsp70) and a biomarker of its inhibition
Abstract
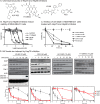
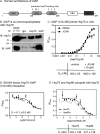
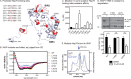
Heat shock protein 70 (Hsp70) and Hsp90 are molecular chaperones that play essential roles in tumor growth by stabilizing pro-survival client proteins. However, although the development of Hsp90 inhibitors has benefited from the identification of clients, such as Raf-1 proto-oncogene, Ser/Thr kinase (RAF1), that are particularly dependent on this chaperone, no equivalent clients for Hsp70 have been reported. Using chemical probes and MDA-MB-231 breast cancer cells, we found here that the inhibitors of apoptosis proteins, including c-IAP1 and X-linked inhibitor of apoptosis protein (XIAP), are obligate Hsp70 clients that are rapidly (within ∼3-12 h) lost after inhibition of Hsp70 but not of Hsp90. Mutagenesis and pulldown experiments revealed multiple Hsp70-binding sites on XIAP, suggesting that it is a direct, physical Hsp70 client. Interestingly, this interaction was unusually tight (∼260 nm) for an Hsp70-client interaction and involved non-canonical regions of the chaperone. Finally, we also found that Hsp70 inhibitor treatments caused loss of c-IAP1 and XIAP in multiple cancer cell lines and in tumor xenografts, but not in healthy cells. These results are expected to significantly accelerate Hsp70 drug discovery by providing XIAP as a pharmacodynamic biomarker. More broadly, our findings further suggest that Hsp70 and Hsp90 have partially non-overlapping sets of obligate protein clients in cancer cells.
Keywords: cancer biology; chaperone; chemical biology; heat shock protein 90 (Hsp90); protein–protein interaction.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Chaperone substrate provides missing link for cancer drug discovery.J Biol Chem. 2018 Feb 16;293(7):2381-2382. doi: 10.1074/jbc.H118.001591. J Biol Chem. 2018. PMID: 29453286 Free PMC article.
-
Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins.J Biol Chem. 2008 Jun 20;283(25):17184-93. doi: 10.1074/jbc.M709447200. Epub 2008 Apr 28. J Biol Chem. 2008. PMID: 18442981
-
Hsp110 chaperones control client fate determination in the hsp70-Hsp90 chaperone system.Mol Biol Cell. 2010 May 1;21(9):1439-48. doi: 10.1091/mbc.e09-09-0779. Epub 2010 Mar 17. Mol Biol Cell. 2010. PMID: 20237159 Free PMC article.
-
Hsp90 and Hsp70 chaperones: Collaborators in protein remodeling.J Biol Chem. 2019 Feb 8;294(6):2109-2120. doi: 10.1074/jbc.REV118.002806. Epub 2018 Nov 6. J Biol Chem. 2019. PMID: 30401745 Free PMC article. Review.
-
The Hsp70-Hsp90 Chaperone Cascade in Protein Folding.Trends Cell Biol. 2019 Feb;29(2):164-177. doi: 10.1016/j.tcb.2018.10.004. Epub 2018 Nov 28. Trends Cell Biol. 2019. PMID: 30502916 Review.
Cited by
-
2-phenylethynesulfonamide inhibits growth of oral squamous cell carcinoma cells by blocking the function of heat shock protein 70.Biosci Rep. 2020 Mar 27;40(3):BSR20200079. doi: 10.1042/BSR20200079. Biosci Rep. 2020. PMID: 32110810 Free PMC article.
-
The Hsp70 inhibitor 2-phenylethynesulfonamide inhibits replication and carcinogenicity of Epstein-Barr virus by inhibiting the molecular chaperone function of Hsp70.Cell Death Dis. 2018 Jun 29;9(7):734. doi: 10.1038/s41419-018-0779-3. Cell Death Dis. 2018. PMID: 29959331 Free PMC article.
-
Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities.MedComm (2020). 2022 Aug 2;3(3):e161. doi: 10.1002/mco2.161. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35928554 Free PMC article. Review.
-
Heat shock factor 1 is a promising therapeutic target against adult T-cell leukemia.Med Oncol. 2023 May 10;40(6):172. doi: 10.1007/s12032-023-02042-5. Med Oncol. 2023. PMID: 37165174
-
Unexpected specificity within dynamic transcriptional protein-protein complexes.Proc Natl Acad Sci U S A. 2020 Nov 3;117(44):27346-27353. doi: 10.1073/pnas.2013244117. Epub 2020 Oct 19. Proc Natl Acad Sci U S A. 2020. PMID: 33077600 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

