Discovery of IDO1 Inhibitors: From Bench to Bedside
- PMID: 29247038
- PMCID: PMC6021761
- DOI: 10.1158/0008-5472.CAN-17-2285
Discovery of IDO1 Inhibitors: From Bench to Bedside
Abstract
Small-molecule inhibitors of indoleamine 2,3-dioxygenase-1 (IDO1) are emerging at the vanguard of experimental agents in oncology. Here, pioneers of this new drug class provide a bench-to-bedside review on preclinical validation of IDO1 as a cancer therapeutic target and on the discovery and development of a set of mechanistically distinct compounds, indoximod, epacadostat, and navoximod, that were first to be evaluated as IDO inhibitors in clinical trials. As immunometabolic adjuvants to widen therapeutic windows, IDO inhibitors may leverage not only immuno-oncology modalities but also chemotherapy and radiotherapy as standards of care in the oncology clinic. Cancer Res; 77(24); 6795-811. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
The authors state a conflict of interest as shareholders, W.J.M. and G.C.P. as compensated scientific advisors, and G.C.P. as a former grant recipient of NewLink Genetics Corporation, based on their roles as inventors of IDO intellectual property licensed to NewLink as described in U.S. Patents Nos. 7705022, 7714139, 8008281, 8058416, 8383613, 8389568, 8436151, 8476454 and 8586636.
Figures

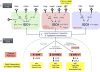
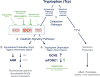



Similar articles
-
A patent review of IDO1 inhibitors for cancer.Expert Opin Ther Pat. 2018 Apr;28(4):317-330. doi: 10.1080/13543776.2018.1441290. Epub 2018 Feb 23. Expert Opin Ther Pat. 2018. PMID: 29473428 Review.
-
Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer.Int Rev Cell Mol Biol. 2018;336:175-203. doi: 10.1016/bs.ircmb.2017.07.004. Epub 2017 Sep 21. Int Rev Cell Mol Biol. 2018. PMID: 29413890 Free PMC article. Review.
-
The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy.Eur J Cancer. 2017 May;76:167-182. doi: 10.1016/j.ejca.2017.01.011. Epub 2017 Mar 18. Eur J Cancer. 2017. PMID: 28324751 Review.
-
Companies Scaling Back IDO1 Inhibitor Trials.Cancer Discov. 2018 Jul;8(7):OF5. doi: 10.1158/2159-8290.CD-ND2018-007. Epub 2018 May 30. Cancer Discov. 2018. PMID: 29848606
-
Discovery of the First Potent IDO1/IDO2 Dual Inhibitors: A Promising Strategy for Cancer Immunotherapy.J Med Chem. 2021 Dec 23;64(24):17950-17968. doi: 10.1021/acs.jmedchem.1c01305. Epub 2021 Dec 2. J Med Chem. 2021. PMID: 34854662
Cited by
-
Hederagenin Attenuates Cerebral Ischaemia/Reperfusion Injury by Regulating MLK3 Signalling.Front Pharmacol. 2020 Jul 30;11:1173. doi: 10.3389/fphar.2020.01173. eCollection 2020. Front Pharmacol. 2020. PMID: 32848779 Free PMC article.
-
Metabolism of immune cells in cancer.Nat Rev Cancer. 2020 Sep;20(9):516-531. doi: 10.1038/s41568-020-0273-y. Epub 2020 Jul 6. Nat Rev Cancer. 2020. PMID: 32632251 Free PMC article. Review.
-
Bypassing evolutionary dead ends and switching the rate-limiting step of a human immunotherapeutic enzyme.Nat Catal. 2022 Oct;5(10):952-967. doi: 10.1038/s41929-022-00856-6. Epub 2022 Oct 19. Nat Catal. 2022. PMID: 36465553 Free PMC article.
-
Prognostic implications of immune classification using IDO1 expression in extrahepatic bile duct carcinoma.Oncol Lett. 2022 Sep 5;24(4):373. doi: 10.3892/ol.2022.13493. eCollection 2022 Oct. Oncol Lett. 2022. PMID: 36238847 Free PMC article.
-
Targeting the immune milieu in gastrointestinal cancers.J Gastroenterol. 2020 Oct;55(10):909-926. doi: 10.1007/s00535-020-01710-x. Epub 2020 Aug 3. J Gastroenterol. 2020. PMID: 32748171 Free PMC article. Review.
References
-
- Prendergast GC, Jaffee EM. Cancer immunologists and cancer biologists: why we didn't talk then but need to now. Cancer Res. 2007;67:3500–4. - PubMed
-
- Prendergast GC, Jaffee EM. Cancer immunotherapy: Immune suppression and tumor growth. 2. New York: Academic Press; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

