mTORC1 Activation during Repeated Regeneration Impairs Somatic Stem Cell Maintenance
- PMID: 29220665
- PMCID: PMC5823264
- DOI: 10.1016/j.stem.2017.11.008
mTORC1 Activation during Repeated Regeneration Impairs Somatic Stem Cell Maintenance
Abstract
The balance between self-renewal and differentiation ensures long-term maintenance of stem cell (SC) pools in regenerating epithelial tissues. This balance is challenged during periods of high regenerative pressure and is often compromised in aged animals. Here, we show that target of rapamycin (TOR) signaling is a key regulator of SC loss during repeated regenerative episodes. In response to regenerative stimuli, SCs in the intestinal epithelium of the fly and in the tracheal epithelium of mice exhibit transient activation of TOR signaling. Although this activation is required for SCs to rapidly proliferate in response to damage, repeated rounds of damage lead to SC loss. Consistently, age-related SC loss in the mouse trachea and in muscle can be prevented by pharmacologic or genetic inhibition, respectively, of mammalian target of rapamycin complex 1 (mTORC1) signaling. These findings highlight an evolutionarily conserved role of TOR signaling in SC function and identify repeated rounds of mTORC1 activation as a driver of age-related SC decline.
Keywords: Drosophila; aging; intestine; mTOR; metabolism; muscle; rapamycin; regeneration; somatic stem cell; trachea.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

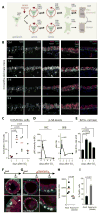

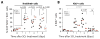
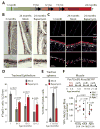
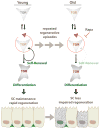
Comment in
-
Regeneration of injured tissue: stem cell dynamics at interplay with mTORC1.Stem Cell Investig. 2018 Aug 30;5:27. doi: 10.21037/sci.2018.08.01. eCollection 2018. Stem Cell Investig. 2018. PMID: 30221172 Free PMC article. No abstract available.
Similar articles
-
Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects.Adv Biol Regul. 2017 May;64:39-48. doi: 10.1016/j.jbior.2016.12.001. Epub 2017 Jan 4. Adv Biol Regul. 2017. PMID: 28189457 Review.
-
Insulin-like Growth Factor-1 and mTORC1 Signaling Promote the Intestinal Regenerative Response After Irradiation Injury.Cell Mol Gastroenterol Hepatol. 2020;10(4):797-810. doi: 10.1016/j.jcmgh.2020.05.013. Epub 2020 Jun 2. Cell Mol Gastroenterol Hepatol. 2020. PMID: 32502530 Free PMC article.
-
Distinct roles of Rheb and Raptor in activating mTOR complex 1 for the self-renewal of hematopoietic stem cells.Biochem Biophys Res Commun. 2018 Jan 1;495(1):1129-1135. doi: 10.1016/j.bbrc.2017.11.140. Epub 2017 Nov 22. Biochem Biophys Res Commun. 2018. PMID: 29175333
-
Prenatal Mechanistic Target of Rapamycin Complex 1 (m TORC1) Inhibition by Rapamycin Treatment of Pregnant Mice Causes Intrauterine Growth Restriction and Alters Postnatal Cardiac Growth, Morphology, and Function.J Am Heart Assoc. 2017 Aug 4;6(8):e005506. doi: 10.1161/JAHA.117.005506. J Am Heart Assoc. 2017. PMID: 28778941 Free PMC article.
-
Aging and stem cell therapy: AMPK as an applicable pharmacological target for rejuvenation of aged stem cells and achieving higher efficacy in stem cell therapy.Hematol Oncol Stem Cell Ther. 2018 Dec;11(4):189-194. doi: 10.1016/j.hemonc.2017.08.001. Epub 2017 Oct 19. Hematol Oncol Stem Cell Ther. 2018. PMID: 29080400 Review.
Cited by
-
mTORC1 signaling pathway regulates tooth repair.Int J Oral Sci. 2023 Mar 16;15(1):14. doi: 10.1038/s41368-023-00218-3. Int J Oral Sci. 2023. PMID: 36927863 Free PMC article.
-
GLI3 regulates muscle stem cell entry into GAlert and self-renewal.Nat Commun. 2022 Jul 8;13(1):3961. doi: 10.1038/s41467-022-31695-5. Nat Commun. 2022. PMID: 35803939 Free PMC article.
-
TAZ stimulates exercise-induced muscle satellite cell activation via Pard3-p38 MAPK-TAZ signalling axis.J Cachexia Sarcopenia Muscle. 2023 Dec;14(6):2733-2746. doi: 10.1002/jcsm.13348. Epub 2023 Nov 3. J Cachexia Sarcopenia Muscle. 2023. PMID: 37923703 Free PMC article.
-
mTOR Signaling at the Crossroad between Metazoan Regeneration and Human Diseases.Int J Mol Sci. 2020 Apr 14;21(8):2718. doi: 10.3390/ijms21082718. Int J Mol Sci. 2020. PMID: 32295297 Free PMC article. Review.
-
Taurine represses age-associated gut hyperplasia in Drosophila via counteracting endoplasmic reticulum stress.Aging Cell. 2021 Mar;20(3):e13319. doi: 10.1111/acel.13319. Epub 2021 Feb 9. Aging Cell. 2021. PMID: 33559276 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

